DPAGT1
UDP-N-acetylglucosamine—dolichyl-phosphate N-acetylglucosaminephosphotransferase is an enzyme that in humans is encoded by the DPAGT1 gene.[5][6]
| DPAGT1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | DPAGT1, ALG7, CDG-Ij, CDG1J, CMSTA2, D11S366, DGPT, DPAGT, DPAGT2, G1PT, GPT, UAGT, UGAT, CMS13, dolichyl-phosphate N-acetylglucosaminephosphotransferase 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 191350 MGI: 1196396 HomoloGene: 1058 GeneCards: DPAGT1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Mutations in DPAGT1 cause myasthenia.[7]
The protein encoded by this gene is an enzyme that catalyzes the first step in the dolichol-linked oligosaccharide pathway (also see Genetic pathway) for glycoprotein biosynthesis. This enzyme belongs to the glycosyltransferase family 4. This protein is an integral membrane protein of the endoplasmic reticulum. The congenital disorder of glycosylation type Ij is caused by mutation in the gene encoding this enzyme. Alternatively spliced transcript variants encoding different isoforms have been identified.[6]
Chemistry
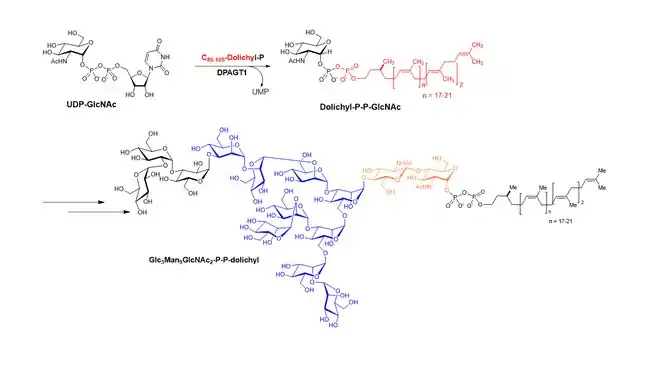
DPAGT1 catalyzes the transformation of dolichyl-phosphate N-acetylglucosamine from Uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) and dolichyl-phosphata, which is the first step in N-glycan biosynthesis in mammalian cells.
Uridine diphosphate N-acetylglucosamine + dolichyl-phosphata ↔ dolichyl-phosphate N-acetylglucosamine + UMP
The generated dolichyl-phosphate N-acetylglucosamine is modified via sequential glycosyltransferases, forming Glc3Man9GlcNAc2-P-P-dolichyl which is used for glycosylation of asparagine (Asn or N) residue of polypeptides.
Structure
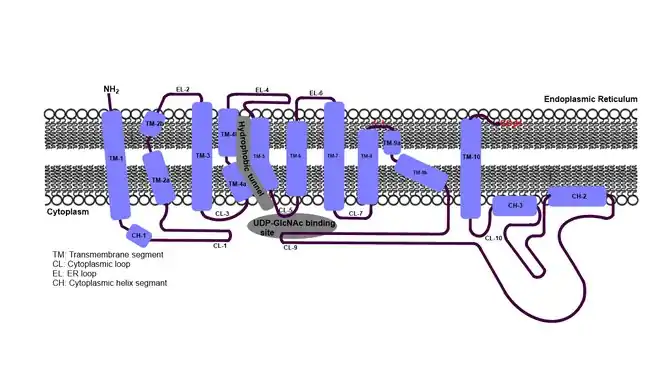
Despite the challenge of obtaining eukaryotic membrane protein structure, co-crystal structures of DPAGT1 with tunicamycin or UDP-GlcNAc have been reported in 2018. [8] [9] DPAGT1 consists of 10 transmembrane segments (TM1 to 10). Three loops on the endoplasmic reticulum (ER) side and five loops on the cytoplasmic side (Loops A-E) connect the transmembrane segments, where TM4, TM5, TM7, TM8, TM9, Loop A, Loop E form the UDP-GlcNAc binding domain. Dolichyl-phosphate (Dol-P) is predicted to bind the “hydrophobic tunnel” created by TM4, TM5 and TM9 within the lipid bilayer. The uridine moiety of tunicamycin occupies the identical binding sites of UDP-GlcNAc. The lipid tail moiety of tunicamycin occupies the hydrophobic tunnel. Significant conformational changes are observed in the C-terminal end of TM-9, Loop A, and Loop E in DPAGT1-ligand bound structures.
Biochemistry
Changes and diversification of the expression profile of cell surface glycans based on the underlying glycobiology have received significant attention from the scientific community. N-Linked and O-linked glycans are the most abundant forms of protein glycosylation and occur on proteins destined for the secretory pathway. Recent studies of cancer immunotherapy are based on the immunogenicity of truncated O-glycan chains (e.g., Tn, sTn, T, and sLea/x). Despite the prevalence of N-linked glycan changes in the development of tumor cells, therapeutic antibodies against N-linked glycans have not been developed. This is likely attributable to the lack of specificity of N-linked glycans between normal and malignant cells. Abnormal branching of N-linked glycans has been observed in certain cancer cells. Altered glycosylation of N-linked glycans in cancers is typically associated with upregulation of ß1,6-N-acetylglucosaminyltransferase-3/5 (GnT3/5), enhancing ß1,6-branching.
References
- GRCh38: Ensembl release 89: ENSG00000172269 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000032123 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Smith MW, Clark SP, Hutchinson JS, Wei YH, Churukian AC, Daniels LB, et al. (September 1993). "A sequence-tagged site map of human chromosome 11". Genomics. 17 (3): 699–725. doi:10.1006/geno.1993.1392. PMID 8244387.
- "Entrez Gene: DPAGT1 dolichyl-phosphate (UDP-N-acetylglucosamine) N-acetylglucosaminephosphotransferase 1 (GlcNAc-1-P transferase)".
- Selcen D, Shen XM, Brengman J, Li Y, Stans AA, Wieben E, Engel AG (May 2014). "DPAGT1 myasthenia and myopathy: genetic, phenotypic, and expression studies". Neurology. 82 (20): 1822–1830. doi:10.1212/WNL.0000000000000435. PMC 4035711. PMID 24759841.
- Yoo J, Mashalidis EH, Kuk AC, Yamamoto K, Kaeser B, Ichikawa S, Lee SY (March 2018). "GlcNAc-1-P-transferase-tunicamycin complex structure reveals basis for inhibition of N-glycosylation". Nature Structural & Molecular Biology. 25 (3): 217–224. doi:10.1038/s41594-018-0031-y. PMC 5840018. PMID 29459785.
- Dong YY, Wang H, Pike AC, Cochrane SA, Hamedzadeh S, Wyszyński FJ, et al. (November 2018). "Structures of DPAGT1 Explain Glycosylation Disease Mechanisms and Advance TB Antibiotic Design". Cell. 175 (4): 1045–1058.e16. doi:10.1016/j.cell.2018.10.037. PMC 6218659. PMID 30388443.
Further reading
- Freeze HH (December 2001). "Update and perspectives on congenital disorders of glycosylation". Glycobiology. 11 (12): 129R–143R. doi:10.1093/glycob/11.12.129R. PMID 11805072.
- Freeze HH (December 2002). "Human disorders in N-glycosylation and animal models". Biochimica et Biophysica Acta (BBA) - General Subjects. 1573 (3): 388–393. doi:10.1016/S0304-4165(02)00408-7. PMID 12417423.
- Miller BS, Freeze HH (March 2003). "New disorders in carbohydrate metabolism: congenital disorders of glycosylation and their impact on the endocrine system". Reviews in Endocrine & Metabolic Disorders. 4 (1): 103–113. doi:10.1023/A:1021883605280. PMID 12618564. S2CID 26028477.
- Volpe JJ, Sakakihara Y, Ishii S (June 1987). "Dolichol-linked glycoprotein synthesis in developing mammalian brain: maturational changes of the N-acetylglucosaminylphosphotransferase". Brain Research. 430 (2): 277–284. doi:10.1016/0165-3806(87)90160-x. PMID 3038274.
- Maruyama K, Sugano S (January 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–174. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Bonaldo MF, Lennon G, Soares MB (September 1996). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Research. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (October 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–156. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Eckert V, Blank M, Mazhari-Tabrizi R, Mumberg D, Funk M, Schwarz RT (January 1998). "Cloning and functional expression of the human GlcNAc-1-P transferase, the enzyme for the committed step of the dolichol cycle, by heterologous complementation in Saccharomyces cerevisiae". Glycobiology. 8 (1): 77–85. doi:10.1093/glycob/8.1.77. PMID 9451016.
- Meissner JD, Naumann A, Mueller WH, Scheibe RJ (March 1999). "Regulation of UDP-N-acetylglucosamine:dolichyl-phosphate N-acetylglucosamine-1-phosphate transferase by retinoic acid in P19 cells". The Biochemical Journal. 338 ( Pt 2) (2): 561–568. doi:10.1042/0264-6021:3380561. PMC 1220086. PMID 10024536.
- Regis S, Dagnino F, Caroli F, Filocamo M (October 2002). "Genomic structure of the human UDP-GlcNAc:dolichol-P GlcNAc-1-P transferase gene". DNA Sequence. 13 (5): 245–250. doi:10.1080/1042517021000017126. PMID 12592703. S2CID 25176842.
- Newell JW, Seo NS, Enns GM, McCraken M, Mantovani JF, Freeze HH (July 2003). "Congenital disorder of glycosylation Ic in patients of Indian origin". Molecular Genetics and Metabolism. 79 (3): 221–228. doi:10.1016/S1096-7192(03)00089-1. PMID 12855228.
- Wu X, Rush JS, Karaoglu D, Krasnewich D, Lubinsky MS, Waechter CJ, et al. (August 2003). "Deficiency of UDP-GlcNAc:Dolichol Phosphate N-Acetylglucosamine-1 Phosphate Transferase (DPAGT1) causes a novel congenital disorder of Glycosylation Type Ij". Human Mutation. 22 (2): 144–150. doi:10.1002/humu.10239. PMID 12872255. S2CID 35331823.