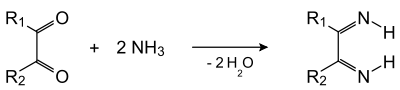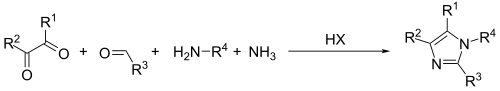Debus–Radziszewski imidazole synthesis
The Debus–Radziszewski imidazole synthesis is a multi-component reaction used for the synthesis of imidazoles from a 1,2-dicarbonyl, an aldehyde, and ammonia or a primary amine. The method is used commercially to produce several imidazoles.[1] The process is an example of a multicomponent reaction.
| Debus–Radziszewski imidazole synthesis | |
|---|---|
| Named after | Heinrich Debus Bronisław Leonard Radziszewski |
| Reaction type | Ring forming reaction |
The reaction can be viewed as occurring in two stages. In the first stage, the dicarbonyl and two ammonia molecules condense with the two carbonyl groups to give a diimine:
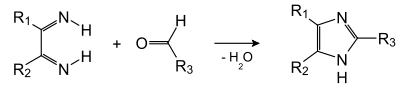
In the second stage, this diimine condenses with the aldehyde:
However, the actual reaction mechanism is not certain.[2][3]
This reaction is named after Heinrich Debus[4] and Bronisław Leonard Radziszewski.[5][6]
A modification of this general method, where one equivalent of ammonia is replaced by an amine, affords N-substituted imidazoles in good yields.[3]
This reaction has been applied to the synthesis of a range of 1,3-dialkylimidazolium ionic liquids by using various readily available alkylamines.[6]
References
- Ebel, K., Koehler, H., Gamer, A. O., & Jäckh, R. "Imidazole and Derivatives." In Ullmann’s Encyclopedia of Industrial Chemistry; 2002 Wiley-VCH, doi:10.1002/14356007.a13_661
- Crouch, R. David; Howard, Jessica L.; Zile, Jennifer L.; Barker, Kathryn H. (2006). "Microwave-Mediated Synthesis of Lophine: Developing a Mechanism To Explain a Product". J. Chem. Educ. 83 (11): 1658–1660. doi:10.1021/ed083p1658.
- Gelens, E.; De Kanter, F. J. J.; Schmitz, R. F.; Sliedregt, L. A. J. M.; Van Steen, B. J.; Kruse, Chris G.; Leurs, R.; Groen, M. B.; Orru, R. V. A. (2006). "Efficient library synthesis of imidazoles using a multicomponent reaction and microwave irradiation". Molecular Diversity. 10: 17–22. doi:10.1007/s11030-006-8695-3.
- Debus, Heinrich (1858). "Ueber die Einwirkung des Ammoniaks auf Glyoxal". Justus Liebigs Annalen der Chemie. 107 (2): 199–208. doi:10.1002/jlac.18581070209.
- Radzisewski, Br. (1882). "Ueber Glyoxalin und seine Homologe". Berichte der deutschen chemischen Gesellschaft. 15 (2): 2706–2708. doi:10.1002/cber.188201502245.
- Damilano, Giacomo; Kalebić, Demian; Binnemans, Koen; Dehaen, Wim (2020). "One-pot synthesis of symmetric imidazolium ionic liquids N,N-disubstituted with long alkyl chains". RSC Adv. 10: 21071–21081. doi:10.1039/D0RA03358H. PMC 9054310.