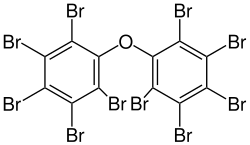
Decabromodiphenyl ether
Decabromodiphenyl ether (also referred to as decaBDE, DBDE, BDE-209) is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs). It was commercialised in the 1970s and was initially thought to be safe,[3][4] but is now recognised as a hazardous and persistent pollutant. It was added to Annex A of the Stockholm Convention on Persistent Organic Pollutants in 2017,[5][6] which means that treaty members must take measures to eliminate its production and use. The plastics industry started switching to decabromodiphenyl ethane as an alternative in the 1990s, but this is now also coming under regulatory pressure due to concerns over human health.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,1′-Oxybis(2,3,4,5,6-pentabromobenzene) | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.013.277 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12Br10O | |
| Molar mass | 959.17 g/mol |
| Appearance | White or pale yellow solid |
| Density | 3.364 g/cm3 solid |
| Melting point | 294 to 296 °C (561 to 565 °F; 567 to 569 K)[1] |
| Boiling point | 425 °C (797 °F; 698 K) (decomposition)[1] |
| 20-30 µg/litre [2] | |
| Hazards | |
| GHS labelling: | |
  | |
| Warning | |
| H302, H312, H319, H332, H341, H373, H413 | |
| P201, P202, P260, P261, P264, P270, P271, P273, P280, P281, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P308+P313, P312, P314, P322, P330, P337+P313, P363, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 241 °C (466 °F; 514 K) |
| Safety data sheet (SDS) | |
| Related compounds | |
Related polybrominated diphenyl ethers |
pentabromodiphenyl ether, octabromodiphenyl ether |
Related compounds |
Diphenyl ether |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Composition, uses, and production
Commercial decaBDE is a technical mixture of various PBDE congeners (related compounds). Congener number 209 (decabromodiphenyl ether) and nonabromodiphenyl ether are the main components.[7] The term decaBDE alone refers to only decabromodiphenyl ether, the single "fully brominated" PBDE.[8]
DecaBDE is a flame retardant. The chemical "is always used in conjunction with antimony trioxide" in polymers, mainly in "high impact polystyrene (HIPS) which is used in the television industry for cabinet backs."[7] DecaBDE is also used for "polypropylene drapery and upholstery fabric" by means of backcoating and "may also be used in some synthetic carpets."[7]
The annual demand worldwide was estimated as 56,100 tonnes in 2001, of which the Americas accounted for 24,500 tonnes, Asia 23,000 tonnes, and Europe 7,600 tonnes.[7] In 2012 between 2500 and 5000 metric tonnes of Deca-BDE was sold in Europe.[9] As of 2007, Albemarle in the U.S., Chemtura in the U.S., ICL-IP in Israel, and Tosoh Corporation in Japan are the main manufacturers of DecaBDE.[10]
Despite its listing in Annex A to the Stockholm Convention, decaBDE is still produced in China, namely in the provinces Shandong and Jiangsu.[11][12]
Environmental chemistry
As stated in a 2006 review, "Deca-BDE has long been characterized as an environmentally stable and inert product that was not capable of degradation in the environment, not toxic, and therefore of no concern."[13] However, "some scientists had not particularly believed that Deca-BDE was so benign, particularly as evidence to this effect came largely from the industry itself."[13] One problem in studying the chemical was that "the detection of Deca-BDE in environmental samples is difficult and problematic"; only in the late 1990s did "analytical advances... allow detection at much lower concentrations."[13]
DecaBDE is released by diverse processes into the environment, such as emissions from manufacture of decaBDE-containing products and from the products themselves.[8] Elevated concentrations can be found in air, water, soil, food, sediment, sludge, and dust.[14] A 2006 study concluded "in general, environmental concentrations of BDE-209 [i.e., decaBDE] appear to be increasing."[14]
The question of debromination
An important scientific issue is whether decaBDE debrominates in the environment to PBDE congeners with fewer bromine atoms, since such PBDE congeners may be more toxic than decaBDE itself.[8] Debromination may be "biotic" (caused by biological means) or "abiotic" (caused by nonbiological means).[10] The European Union (EU) in May 2004 stated "the formation of PBT/vPvB (Persistent, Bioaccumulative, and Toxic / very Persistent, very Bioaccumulative) substances in the environment as a result of degradation [of decaBDE] is a possibility that cannot be quantified based on current knowledge."[7] In September 2004 an Agency for Toxic Substances and Disease Registry (ATSDR) report asserted that "DecaBDE seems to be largely resistant to environmental degradation."[8]
In May 2006, the EPHA Environment Network (now The Health and Environment Alliance) released a report reviewing the available scientific literature[15] and concluding the following:
- "It is difficult to assess the degree of BDE 209 photolytic debromination in house dust, soils and sediments when exposed to light. However, in cars debromination can be expected to occur more significantly."
- "In sewage anaerobic bacteria can initiate debromination of BDE 209, albeit at a slower rate than photolytic debromination, but due to the large volumes of DecaBDE in sewage sludge this may be significant."
- "Some fish appear capable of debrominating BDE 209 through metabolism. The extent of the metabolism varies among fish and it is difficult to determine the extent of debromination that would occur in the wild."
Subsequently, many studies have been published concerning decaBDE debromination. Common anaerobic soil bacteria debrominated decaBDE and octaBDE in a 2006 study.[16] In 2006-2007 studies, metabolic debromination of decaBDE was demonstrated in fish,[17] birds,[18] cows,[19] and rats.[20] A 2007 study by La Guardia and colleagues measured PBDE congeners "from a wastewater treatment plant (sludge) to receiving stream sediments and associated aquatic biota"; it "support[ed] the hypothesis that metabolic debromination of -209 [i.e., decaBDE] does occur in the aquatic environment under realistic conditions."[21] In another 2007 study, Stapleton and Dodder exposed "both a natural and a BDE 209 spiked [house] dust material" to sunlight, and found "nonabrominated congeners" and "octabrominated congeners" consistent with debromination of decaBDE in the environment.[22]
In March 2007 the Illinois Environmental Protection Agency concluded "it can be questioned how much abiotic and microbial degradation [of decaBDE] occurs under normal environmental conditions, and it is not clear whether the more toxic lower-brominated PBDEs are produced in significant quantities by any of these pathways."[23] In September 2010, the UK Advisory Committee on Hazardous Substances issued an opinion that ‘there is strong but incomplete, scientific evidence indicating that Deca-BDE has the potential to undergo transformation to lower brominated congeners in the environment'.[24]
Pharmacokinetics
Exposure to decaBDE is thought to occur by means of ingestion.[8] Humans and animals do not absorb decaBDE well; at most, perhaps 2% of an oral dose is absorbed.[25][26] It is believed that "the small amount of decaBDE that is absorbed can be metabolized".[8]
Once in the body, decaBDE "might leave unchanged or as metabolites, mainly in the feces and in very small amounts in the urine, within a few days," in contrast with "lower brominated PBDEs... [which] might stay in your body for many years, stored mainly in body fat."[8] In workers with occupational exposure to PBDEs, the calculated apparent half-life for decaBDE was 15 days, as opposed to (for example) an octaBDE congener with a half-life of 91 days.[27]
Detection in humans
In the general population, decaBDE has been found in blood and breast milk, but at lower levels than other PBDE congeners such as 47, 99, and 153.[28] An investigation carried out by the WWF detected decaBDE in blood samples from 3 of 14 ministers of health and environment of European Union countries, while (for example) PBDE-153 was found in all 14.[29]
Possible health effects in humans
In 2004, ATSDR wrote "Nothing definite is known about the health effects of PBDEs in people. Practically all of the available information is from studies of laboratory animals. Animal studies indicate that commercial decaBDE mixtures are generally much less toxic than the products containing lower brominated PBDEs. DecaBDE is expected to have relatively little effect on the health of humans."[8] Based on animal studies, the possible health effects of decaBDE in humans involve the liver, thyroid, reproductive/developmental effects, and neurological effects.[30]
Liver
ATSDR stated in 2004 "We don’t know if PBDEs can cause cancer in people, although liver tumors developed in rats and mice that ate extremely large amounts of decaBDE throughout their lifetime. On the basis of evidence for cancer in animals, decaBDE is classified as a possible human carcinogen by EPA [i.e., the United States Environmental Protection Agency ]."[8]
Thyroid
One 2006 review concluded "Decreases in thyroid hormone levels have been reported in several studies, and thyroid gland enlargement (an early sign of hypothyroidism) has been shown in studies of longer duration exposure."[30] A 2007 experiment giving decaBDE to pregnant mice found that decaBDE "is likely an endocrine disrupter in male mice following exposure during development" based on results such as decreased serum triiodothyronine.[31]
Reproductive/developmental effects
"Significant data gaps" exist in the scientific literature on a possible relationship between decaBDE and reproductive/developmental effects.[30] A 2006 study of mice found that decaBDE decreased some "sperm functions."[32]
Neurological effects
EPA has determined that daily Deca exposures should be less than 7 μg/kg-d (micrograms per kilogram bodyweight per day) to minimize the chance of brain and nervous system toxicity.[33] EPA based their assessment on a study in 2003 on neurotoxicity in mice, which some have "criticized for certain procedural and statistical problems."[30] A 2007 study in mice "suggest[ed] that decaBDE is a developmental neurotoxicant that can produce long-term behavioral changes following a discrete period of neonatal exposure."[34] Administration of decaBDE to male rats at 3 days of age in another 2007 study "was shown to disrupt normal spontaneous behaviour at 2 months of age."[35]
Overall risks and benefits
In 2002–2003 the American Chemistry Council's Brominated Flame Retardant Industry Panel, citing an unpublished 1997 study, estimated that 280 deaths due to fires are prevented each year in the U.S. because of the use of decaBDE.[25][26] The industry advocacy group American Council on Science and Health, in a 2006 report largely concerning decaBDE, said that "the benefits of PBDE flame retardants, in terms of lives saved and injuries prevented, far outweigh any demonstrated or likely negative health effects from their use."[36] A 2006 study concluded "current levels of Deca in the United States are unlikely to represent an adverse health risk for children."[37] A report from the Swedish National Testing and Research Institute concerning the costs and benefits of decaBDE in television sets that was funded by BSEF assumed "no cost for injuries (either to humans or the environment) due to exposure to flame retardants... as there was no indication that such costs exist for DecaBDE"; it found that decaBDE's benefits exceeded its costs.[38]
Voluntary and governmental actions
Europe
In Germany, plastics manufacturers and the textile additives industry "declared in 1986 a voluntary phase-out of the use of PBDEs, including Deca-BDE."[39] Although decaBDE was to be phased out of electrical and electronic equipment in the EU by 2006 under the EU's Restriction of Hazardous Substances Directive (RoHS), decaBDE use has been exempted from RoHS during 2005–2010.[40][41][42] A case in the European Court of Justice against the RoHS exemption was decided against Deca-BDE and its use must be phased out by July 1, 2008.[10] Sweden, an EU member, banned decaBDE as of 2007.[28][43] The former European Brominated Flame Retardant Industry Panel (EBFRIP), now merged with EFRA, the European Flame Retardant Association, stated that Sweden's ban on DecaBDE "was a serious breach of EU law. . The European Commission then started an infringement procedure against Sweden which lead to the Swedish Government repealing this restriction on 1 July 2008 . The environment agency of Norway, which is a member of the European Free Trade Association but is not a member of the EU, recommended that decaBDE be banned from electronic products in 2008.[44]
DecaBDE has been the subject of a ten-year evaluation under the EU Risk Assessment procedure which has reviewed over 1100 studies. The Risk Assessment was published on the EU Official Journal in May 2008.[45] Deca was registered under the EU's REACH Regulation at the end of August 2010.
The UK's Advisory Committee on Hazardous Substances (ACHS) presented their conclusions following a review of the emerging studies on Deca-BDE on 14 September 2010.
On 5 July ECHA withdrew Deca-BDE from its list of priority substances for Authorisation under REACH, therefore closing the public consultation. On 1 August 2014, ECHA submitted a restriction proposal for Deca-BDE. The agency is proposing a restriction on the manufacture, use and placing on the market of the substance and of mixtures and articles containing it. On 17 September 2014, ECHA submitted the restriction report which initiates a six months public consultation. On 9 February 2017, the European Commission adopted Regulation EU 2017/227. Article 1 of this regulation states that Regulation (EC) No 1907/2006 is amended to include a ban on the use of decaBDE in quantities greater than 0.1% by weight, effective from 2 March 2019. Products placed on the market prior to 2 March 2019 are exempt. Furthermore, the use decaBDE in aircraft is permissible until 2 March 2027.[46] This EU process is running in parallel with a UNEP review to determine whether Deca-BDE should be listed as a Persistent Organic Pollutant (POP) under the Stockholm Convention.
United States
As of mid-2007 two states had instituted measures to phase out decaBDE. In April 2007 the state of Washington passed a law banning the manufacture, sale, and use of decaBDE in mattresses as of 2008; the ban "could be extended to TVs, computers and upholstered residential furniture in 2011 provided an alternative flame retardant is approved."[47][48] In June 2007 the state of Maine passed a law "ban[ning] the use of deca-BDE in mattresses and furniture on January 1, 2008 and phas[ing] out its use in televisions and other plastic-cased electronics by January 1, 2010."[49][50] As of 2007, other states considering restrictions on decaBDE include California, Connecticut, Hawaii, Illinois, Massachusetts, Michigan, Minnesota,[51] Montana, New York, and Oregon.[43][52]
On December 17, 2009, as the result of negotiations with EPA, the two U.S. producers of decabromodiphenyl ether (decaBDE), Albemarle Corporation and Chemtura Corporation, and the largest U.S. importer, ICL Industrial Products, Inc., announced commitments to phase out voluntarily decaBDE in the United States by the end of 2013. Archived 2016-01-19 at the Wayback Machine, ,
Alternatives
A number of reports have examined alternatives to decaBDE as a flame retardant.[39][53][54][55][56][57] At least three U.S. states have evaluated decaBDE alternatives:
- Washington concluded in 2006 that "there do not appear to be any obvious alternatives to Deca-BDE that are less toxic, persistent and bioaccumulative and have enough data available for making a robust assessment" and that "there is much more data available on Deca-BDE than for any of the alternatives."[58]
- Maine in January 2007 stated that bisphenol A diphenyl phosphate (also known as BDP, BPADP, bisphenol A diphosphate, or BAPP) "is not a suitable alternative to decaBDE" because "one of the degradation products is bisphenol A, a potent endocrine disruptor."[10] The report listed resorcinol bis(diphenyl phosphate) (also known as RDP), magnesium hydroxide, and other chemicals as alternatives to decaBDE that are "most likely to be used."[10]
- A March 2007 report from Illinois categorized decaBDE alternatives as "Potentially Unproblematic," "Potentially Problematic," "Insufficient Data," and "Not Recommended."[23] The "Potentially Unproblematic" alternatives were BAPP, RDP, aluminum trihydroxide, and magnesium hydroxide.[23]
References
- Record of Decabromodiphenyl ether in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 17 June 2008.
- "Environmental Health Criteria". ICPS. Retrieved 2009-07-20.
- Liepins, R; Pearce, E M (October 1976). "Chemistry and toxicity of flame retardants for plastics". Environmental Health Perspectives. 17: 55–63. doi:10.1289/ehp.761755. PMC 1475265. PMID 1026419.
- Norris, J M; Kociba, R J; Schwetz, B A; Rose, J Q; Humiston, C G; Jewett, G L; Gehring, P J; Mailhes, J B (June 1975). "Toxicology of octabromobiphenyl and decabromodiphenyl oxide". Environmental Health Perspectives. 11: 153–161. doi:10.1289/ehp.7511153. PMC 1475203. PMID 126149.
- "c-decaBDE". chm.pops.int. Secretariat of the Stockholm Convention. Retrieved 8 January 2023.
- Reference: C.N.766.2017.TREATIES-XXVII.15 (Depositary Notification)
- Joint Research Centre European inventory of Existing Commercial chemical Substances "Archived copy". Archived from the original on 2014-01-01. Retrieved 2009-07-11.
{{cite web}}: CS1 maint: archived copy as title (link) - Agency for Toxic Substances and Disease Registry. Toxicological Profile for Polybrominated Biphenyls and Polybrominated Diphenyl Ethers (PBBs and PBDEs). Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, September 2004.
- "VECAP - Annual Progress Report".
- Brominated Flame Retardants: Third annual report to the Maine Legislature. Archived 2007-03-11 at the Wayback Machine Augusta, Maine: Maine Department of Environmental Protection and Maine Center for Disease Control & Prevention, January 2007.
- Chen, Yuan; Li, Jinhui; Tan, Quanyin (2020-05-01). "Trends of production, consumption and environmental emissions of Decabromodiphenyl ether in mainland China". Environmental Pollution. 260: 114022. doi:10.1016/j.envpol.2020.114022. ISSN 0269-7491. PMID 31995770. S2CID 210951187.
- Zhen, Xiaomei; Li, Yanfang; Tang, Jianhui; Wang, Xinming; Liu, Lin; Zhong, Mingyu; Tian, Chongguo (2021-05-17). "Decabromodiphenyl Ether versus Decabromodiphenyl Ethane: Source, Fate, and Influencing Factors in a Coastal Sea Nearing Source Region". Environmental Science & Technology. 55 (11): 7376–7385. Bibcode:2021EnST...55.7376Z. doi:10.1021/acs.est.0c08528. ISSN 0013-936X. PMID 33998794. S2CID 234747918.
- Alcock RE, Busby J (April 2006). "Risk migration and scientific advance: the case of flame-retardant compounds". Risk Anal. 26 (2): 369–81. doi:10.1111/j.1539-6924.2006.00739.x. PMID 16573627. S2CID 37119476.
- Hale RC, La Guardia MJ, Harvey E, Gaylor MO, Mainor TM (June 2006). "Brominated flame retardant concentrations and trends in abiotic media". Chemosphere. 64 (2): 181–6. Bibcode:2006Chmsp..64..181H. doi:10.1016/j.chemosphere.2005.12.006. PMID 16434082.
- Stapleton, Heather M. Brominated Flame Retardants: Assessing DecaBDE Debromination in the Environment. Brussels, Belgium: EPHA Environment Network, May 2006.
- He J, Robrock KR, Alvarez-Cohen L (July 2006). "Microbial reductive debromination of polybrominated diphenyl ethers (PBDEs)". Environ. Sci. Technol. 40 (14): 4429–34. Bibcode:2006EnST...40.4429H. doi:10.1021/es052508d. PMID 16903281.
- Stapleton HM, Brazil B, Holbrook RD, et al. (August 2006). "In vivo and in vitro debromination of decabromodiphenyl ether (BDE 209) by juvenile rainbow trout and common carp". Environ. Sci. Technol. 40 (15): 4653–8. Bibcode:2006EnST...40.4653S. doi:10.1021/es060573x. PMID 16913120.
- Van den Steen E (July 2007). "Accumulation, tissue-specific distribution and debromination of decabromodiphenyl ether (BDE 209) in European starlings (Sturnus vulgaris)". Environ. Pollut. 148 (2): 648–53. doi:10.1016/j.envpol.2006.11.017. PMID 17239511.
- Kierkegaard A, Asplund L, de Wit CA, et al. (January 2007). "Fate of higher brominated PBDEs in lactating cows". Environ. Sci. Technol. 41 (2): 417–23. Bibcode:2007EnST...41..417K. doi:10.1021/es0619197. PMID 17310701.
- Huwe JK, Smith DJ (April 2007). "Accumulation, whole-body depletion, and debromination of decabromodiphenyl ether in male sprague-dawley rats following dietary exposure". Environ. Sci. Technol. 41 (7): 2371–7. Bibcode:2007EnST...41.2371H. doi:10.1021/es061954d. PMID 17438789.
- La Guardia MJ, Hale RC, Harvey E (October 2007). "Evidence of debromination of decabromodiphenyl ether (BDE-209) in biota from a wastewater receiving stream". Environ. Sci. Technol. 41 (19): 6663–70. Bibcode:2007EnST...41.6663L. doi:10.1021/es070728g. PMID 17969678.
- Stapleton HM, Dodder NG (February 2008). "Photodegradation of decabromodiphenyl ether in house dust by natural sunlight". Environ. Toxicol. Chem. 27 (2): 306–12. doi:10.1897/07-301R.1. PMID 18348638. S2CID 207267052.
- Illinois Environmental Protection Agency. Report on Alternatives to the Flame Retardant DecaBDE: Evaluation of Toxicity, Availability, Affordability, and Fire Safety Issues. March 2007.
- Advisory Committee on Hazardous Substances. ACHS opinion on the Draft Environmental Risk Assessment Report for Decabromodiphenyl ether (DecaBDE) (CAS 1163 19 5). Department for Environment, Food and Rural Affairs
- Voluntary Children’s Chemical Evaluation Program (VCCEP) Data Summary: Decabromodiphenyl Ether (a.k.a. Decabromodiphenyl Oxide, DBDPO) CAS # 1163-19-5. Archived 2005-02-20 at the Wayback Machine American Chemistry Council’s Brominated Flame Retardant Industry Panel (BFRIP), December 17, 2002.
- Report of the Peer Consultation Meeting On Decabromodiphenyl Ether. Archived 2006-09-27 at the Wayback Machine American Chemistry Council’s Brominated Flame Retardant Industry Panel for the Voluntary Children's Chemical Evaluation Program (VCCEP), September 30, 2003.
- Thuresson K, Höglund P, Hagmar L, Sjödin A, Bergman A, Jakobsson K (February 2006). "Apparent Half-Lives of Hepta- to Decabrominated Diphenyl Ethers in Human Serum as Determined in Occupationally Exposed Workers". Environ. Health Perspect. 114 (2): 176–81. doi:10.1289/ehp.8350. PMC 1367828. PMID 16451851.
- Lorber M (January 2008). "Exposure of Americans to polybrominated diphenyl ethers". J Expo Sci Environ Epidemiol. 18 (1): 2–19. doi:10.1038/sj.jes.7500572. PMID 17426733.
- WWF Detox Campaign. Bad Blood? A Survey of Chemicals in the Blood of European Ministers. October 2004.
- Illinois Environmental Protection Agency. DecaBDE Study: A Review of Available Scientific Research. January 2006.
- Tseng LH, Li MH, Tsai SS, et al. (January 2008). "Developmental exposure to decabromodiphenyl ether (PBDE 209): effects on thyroid hormone and hepatic enzyme activity in male mouse offspring" (PDF). Chemosphere. 70 (4): 640–7. Bibcode:2008Chmsp..70..640T. doi:10.1016/j.chemosphere.2007.06.078. PMID 17698168.
- Tseng LH, Lee CW, Pan MH, et al. (July 2006). "Postnatal exposure of the male mouse to 2,2',3,3',4,4',5,5',6,6'-decabrominated diphenyl ether: decreased epididymal sperm functions without alterations in DNA content and histology in testis" (PDF). Toxicology. 224 (1–2): 33–43. doi:10.1016/j.tox.2006.04.003. PMID 16713668.
- EPA. 2008. Final Integrated Risk Information System Assessment for Decabromodiphenyl ether (BDE-209). Available: http://www.epa.gov/ncea/iris/subst/0035.htm
- Rice DC, Reeve EA, Herlihy A, Zoeller RT, Thompson WD, Markowski VP (2007). "Developmental delays and locomotor activity in the C57BL6/J mouse following neonatal exposure to the fully-brominated PBDE, decabromodiphenyl ether". Neurotoxicol Teratol. 29 (4): 511–20. doi:10.1016/j.ntt.2007.03.061. PMID 17482428.
- Viberg H, Fredriksson A, Eriksson P (January 2007). "Changes in spontaneous behaviour and altered response to nicotine in the adult rat, after neonatal exposure to the brominated flame retardant, decabrominated diphenyl ether (PBDE 209)". Neurotoxicology. 28 (1): 136–42. doi:10.1016/j.neuro.2006.08.006. PMID 17030062.
- Kucewicz, William P. Brominated Flame Retardants: A Burning Issue. Archived 2011-05-21 at the Wayback Machine New York: American Council on Science and Health, August 2006.
- Hays SM, Pyatt DW (January 2006). "Risk assessment for children exposed to decabromodiphenyl (oxide) ether (Deca) in the United States". Integr Environ Assess Manag. 2 (1): 2–12. doi:10.1897/1551-3793(2006)2[2:RAFCET]2.0.CO;2. PMID 16640311. S2CID 23777908.
- Simonson, Margaret, et al. Cost Benefit Analysis Model for Fire Safety Methodology and TV (DecaBDE) Case Study. Archived 2008-12-06 at the Wayback Machine Swedish National Testing and Research Institute, SP Report 2006:28.
- Lassen, Carsten, et al. Deca-BDE and Alternatives in Electrical and Electronic Equipment. Danish Environmental Protection Agency, 2006.
- COMMISSION DECISION of 13 October 2005 amending for the purposes of adapting to the technical progress the Annex to Directive 2002/95/EC of the European Parliament and of the Council on the restriction of the use of certain hazardous substances in electrical and electronic equipment (2005/717/EC). Official Journal of the European Union 15.10.2005.
- COMMISSION DECISION of 24 September 2010 amending, for the purposes of adapting to scientific and technical progress, the Annex to Directive 2002/95/EC of the European Parliament and of the Council as regards exemptions for applications containing lead, mercury, cadmium, hexavalent chromium, polybrominated biphenyls or polybrominated diphenyl ethers (2010/571/EU). Official Journal of the European Union 24.09.2010.
- Washington State Departments of Health and Ecology. Developments within the European Union Regarding Deca-BDE as Interpreted by Health and Ecology Staff. October 12, 2005.
- Stiffler, Lisa. PBDEs: They are everywhere, they accumulate and they spread. Seattle Post-Intelligencer, March 28, 2007.
- Norwegian Ban on Deca PBDE "" Norwegian Pollution Control Agency.
- Official Journal of the European Union: Communication from the Commission on the results of the risk evaluation of chlorodifluoromethane, bis(pentabromophenyl)ether and methenamine and on the risk reduction strategy for the substance methenamine, 29.5.2008
- The European Commission (9 February 2017). "Commission Regulation (EU) 2017/227". Official Journal of the European Union. L35: 6–9. Retrieved 16 June 2017.
- Stiffler, Lisa. Chemical ban puts industry on the defensive. Seattle Post-Intelligencer, April 16, 2007.
- "Archived copy" (PDF). Archived from the original (PDF) on 2015-04-02. Retrieved 2012-12-15.
{{cite web}}: CS1 maint: archived copy as title (link) - Maine Legislature votes to ban toxic Deca flame retardant. Natural Resources Council of Maine, May 24th, 2007.
- Maine House Democrats. Governor signs deca ban bill into law: State will require phase-out of the flame retardant in household items. Archived 2007-06-30 at the Wayback Machine June 14, 2007.
- SF0651 Status in Senate for Legislative Session 85
- Maine Joins Washington, Bans PBDEs. Archived 2007-08-02 at the Wayback Machine Washington, DC: National Caucus of Environmental Legislators, June 18, 2007.
- Leisewitz, André, et al. Substituting Environmentally Relevant Flame Retardants: Assessment Fundamentals: Results and summary overview. Archived 2011-06-10 at the Wayback Machine Berlin, Germany: Federal Environmental Agency (Umweltbundesamt), June 2001.
- Pure Strategies, Inc. Decabromodiphenylether: An Investigation of Non-Halogen Substitutes in Electronic Enclosure and Textile Applications. Lowell, MA: University of Massachusetts Lowell, Lowell Center for Sustainable Production, April 2005.
- Posner, Stefan, and Linda Börås. Survey and technical assessment of alternatives to Decabromodiphenyl ether (DecaBDE) in plastics. Archived 2007-10-24 at the Wayback Machine Stockholm: Swedish Chemicals Inspectorate, June 2005.
- Stuer-Lauridsen, Frank, et al. Health and Environmental Assessment of Alternatives to Deca-BDE in Electrical and Electronic Equipment. Danish Environmental Protection Agency, 2007.
- Pakalin, Sazan, et al. Review on production processes of decabromodiphenyl ether (decaBDE) used in polymeric applications in electrical and electronic equipment, and assessment of the availability of potential alternatives to decaBDE. Archived 2008-05-12 at the Wayback Machine European Chemicals Bureau, January 2007.http://publications.jrc.ec.europa.eu/repository/handle/111111111/5259
- Washington State Polybrominated Diphenyl Ether (PBDE) Chemical Action Plan: Final Plan. Archived 2007-02-09 at the Wayback Machine Washington State Departments of Ecology and Health, January 19, 2006.
