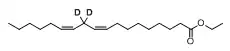
Di-deuterated linoleic acid ethyl ester
Di-deuterated ethyl linoleate (also known as RT001, di-deuterated linoleic acid ethyl ester, 11,11-d2-ethyl linoleate, or ethyl 11,11-d2-linoleate)[1] is an experimental, orally-bioavailable synthetic deuterated polyunsaturated fatty acid (PUFA), a part of reinforced lipids. It is an isotopologue of linoleic acid, an essential omega-6 PUFA. The deuterated compound, while identical to natural linoleic acid except for the presence of deuterium, is resistant to lipid peroxidation which makes studies of its cell-protective properties worthwhile.
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C20H34D2O2 |
| Molar mass | 310.517 g·mol−1 |
| 3D model (JSmol) | |
| Density | 0.88 g/cm3 |
| Boiling point | 173–177 °C (343–351 °F) |
| |
| |
| | |
Mechanism of action
Di-deuterated linoleic acid is recognized by cells as identical to the natural linoleic acid. But when taken up, it is converted into 13,13-d2-arachidonic acid, a heavy isotope version of arachidonic acid, that gets incorporated into lipid membranes. The deuterated compound resists the non-enzymatic lipid peroxidation (LPO) through isotope effect — a non-antioxidant based mechanism that protects mitochondrial, neuronal and other lipid membranes, thereby greatly reducing the levels of numerous LPO-derived toxic products such as reactive carbonyls.[2][3]
Di-deuterated linoleic acid (RT001) inhibits ferroptosis by stopping the autoxidation process through the kinetic isotope effect. The protective effect of D-PUFAs was verified in erastin- and RSL3-induced ferroptosis models, with demonstrated efficacy in various disease models, particularly neurodegenerative disorders and clinical trials of RT001 begun in 2018.[4]
Clinical development
Friedreich's ataxia
A double-blind comparator-controlled Phase I/II clinical trial for Friedreich's ataxia, sponsored by Retrotope and Friedreich's Ataxia Research Alliance, was conducted to determine the safety profile and appropriate dosing for consequent trials.[5] RT001 was promptly absorbed and was found to be safe and tolerable over 28 days at the maximal dose of 9 g/day. It improved peak workload and peak oxygen consumption in the test group compared to the control group who received the equal doses of normal, non-deuterated ethyl linoleate.[6] Another randomised, double-blind, placebo-controlled clinical study began in 2019.[7]
Infantile neuroaxonal dystrophy
An open-label clinical study for infantile neuroaxonal dystrophy evaluating long-term evaluation of efficacy, safety, tolerability, and pharmacokinetics of RT001, which, when taken with food, can protect the neuronal cells from degeneration, started in the Summer 2018.[8]
Phospholipase 2G6-associated neurodegeneration
In 2017, the FDA granted RT001 orphan drug designation in the treatment of phospholipase 2G6-associated neurodegeneration (PLAN).[9]
Amyotrophic lateral sclerosis
In 2018, RT001 was given to a patient with amyotrophic lateral sclerosis (ALS) under a "compassionate use scheme".[10]
Progressive supranuclear palsy
In 2020, the FDA granted orphan drug designation RT001 for the treatment of patients with progressive supranuclear palsy (PSP). PSP is a disease involving modification and dysfunction of tau protein; RT001’s mechanism of action both lowers lipid peroxidation and prevents mitochondrial cell death of neurons which is associated with disease onset and progression.[11]
Preclinical research
Alzheimer's disease
RT001 has been shown to be effective in a model of Alzheimer's disease in mice.[12]
References
- "9-cis, 12-cis-11,11-D2-Linoleic acid ethyl ester". PubChem.
- Hill S, Lamberson CR, Xu L, To R, Tsui HS, Shmanai VV, et al. (August 2012). "Small amounts of isotope-reinforced polyunsaturated fatty acids suppress lipid autoxidation". Free Radical Biology & Medicine. 53 (4): 893–906. doi:10.1016/j.freeradbiomed.2012.06.004. PMC 3437768. PMID 22705367.
- Demidov VV (August 2020). "Site-specifically deuterated essential lipids as new drugs against neuronal, retinal and vascular degeneration". Drug Discovery Today. 25 (8): 1469–1476. doi:10.1016/j.drudis.2020.03.014. PMID 32247036. S2CID 214794450.
- Scarpellini, Camilla; Klejborowska, Greta; Lanthier, Caroline; Hassannia, Behrouz; Vanden Berghe, Tom; Augustyns, Koen (2023). "Beyond ferrostatin-1: a comprehensive review of ferroptosis inhibitors". Trends in Pharmacological Sciences: S0165–6147(23)00182–7. doi:10.1016/j.tips.2023.08.012. PMID 37770317.
- Clinical trial number NCT02445794 for "A First in Human Study of RT001 in Patients With Friedreich's Ataxia" at ClinicalTrials.gov
- Zesiewicz T, Heerinckx F, De Jager R, Omidvar O, Kilpatrick M, Shaw J, Shchepinov MS (July 2018). "Randomized, clinical trial of RT001: Early signals of efficacy in Friedreich's ataxia". Movement Disorders. 33 (6): 1000–1005. doi:10.1002/mds.27353. PMID 29624723. S2CID 4664990.
- Clinical trial number NCT04102501 for "A Study to Assess Efficacy, Long Term Safety and Tolerability of RT001 in Subjects With Friedreich's Ataxia" at ClinicalTrials.gov
- Clinical trial number NCT03570931 for "A Study to Assess Efficacy and Safety of RT001 in Subjects With Infantile Neuroaxonal Dystrophy" at ClinicalTrials.gov
- "US FDA Grants Orphan Drug Designation for Retrotope's RT001 in the Treatment of Phospholipase 2G6 (PLA2G6)-Associated Neurodegeneration". Global Newswire. 2 November 2017.
- Inacio P (2018-09-18). "Experimental RT001 Now Available for ALS Under Expanded Access". ALS News Today.
- "RT001 Gets Orphan Drug Designation in Progressive Supranuclear Palsy".
- Butterfield DA, Halliwell B (March 2019). "Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease". Nature Reviews. Neuroscience. 20 (3): 148–160. doi:10.1038/s41583-019-0132-6. PMC 9382875. PMID 30737462. S2CID 59617957.