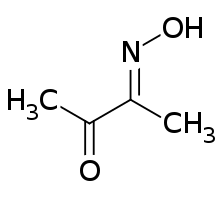
Diacetyl monoxime
Diacetyl monoxime is a chemical compound described by the formula CH3C(O)C(NOH)CH3. This colourless solid is the monooxime derivative of the diketone butane-2,3-dione (also known as diacetyl and biacetyl). Its biological effects include inhibiting certain ATPases.[1]
 | |
| Names | |
|---|---|
| Other names
BDM, Biacetyl monoxime, 2,3-butanedione monoxime | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.316 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H7NO2 | |
| Molar mass | 101.105 g·mol−1 |
| Appearance | white solid |
| Melting point | 75–78 °C (167–172 °F; 348–351 K) |
| Boiling point | 185–186 °C (365–367 °F; 458–459 K) |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H312, H315, H319, H332, H335 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation
The compound can be prepared from butanone by reaction with ethyl nitrite. It is an intermediate in the preparation of dimethylglyoxime:[2]
Uses
Diacetyl monoxime can be used with thiosemicarbazide to selectively detect small amounts of urea in the presence of other nitrogen-containing compounds.[3]
References
- Forer, Arthur; Fabian, Lacramioara (2005). "Does 2,3-butanedione monoxime inhibit nonmuscle myosin?". Protoplasma. 225 (1–2): 1–4. doi:10.1007/s00709-004-0077-z. PMID 15868207. S2CID 10475777.
- Semon, W. L.; Damerell, V. R. (1930). "Dimethylglyoxime". Organic Syntheses. 10: 22. doi:10.15227/orgsyn.010.0022.
- Douglas, L. A.; Bremner, J. M. (1970-02-01). "Colorimetric Determination of Microgram Quantities of Urea". Analytical Letters. 3 (2): 79–87. doi:10.1080/00032717008067782. ISSN 0003-2719.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
