Diazanaphthalene
Diazanaphthalenes are a class of aromatic heterocyclic chemical compounds that have the formula C8H6N2. They consist of a naphthalene double ring in which two of the carbon atoms have been replaced with nitrogen atoms. There are ten positional isomers, which differ by the locations of the nitrogen atoms.
The group consist of two subgroups:
- four benzodiazines with both N atoms in one ring: cinnoline, quinazoline, quinoxaline, and phthalazine
- six naphthyridines with one N atom in each ring[1][2]
Isomers
| Names | Structure | Subgroup | CAS RN |
|---|---|---|---|
| 1,2-diazanaphthalene (cinnoline) | 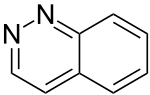 | benzodiazine | 253-66-7 |
| 2,3-diazanaphthalene (phthalazine) | 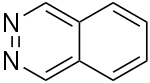 | benzodiazine | 253-52-1 |
| 1,3-diazanaphthalene (quinazoline) | 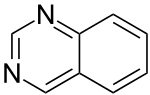 | benzodiazine | 253-82-7 |
| 1,4-diazanaphthalene (quinoxaline) | 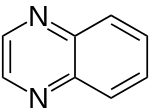 | benzodiazine | 91-19-0 |
| 1,7-naphthyridine (1,7-diazanaphthalene) | 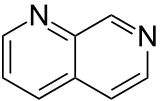 | naphthyridine | 253-69-0 |
| 1,8-Naphthyridine (1,8-diazanaphthalene) | 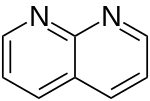 | naphthyridine | 254-60-4 |
| 2,7-naphthyridine (2,7-diazanaphthalene) | 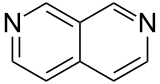 | naphthyridine | 253-45-2 |
| 1,6-naphthyridine (1,6-diazanaphthalene) | 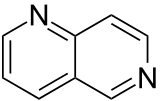 | naphthyridine | 253-72-5 |
| 1,5-naphthyridine (1,5-diazanaphthalene) | 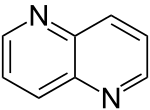 | naphthyridine | 254-79-5 |
| 2,6-naphthyridine (2,6-diazanaphthalene) | 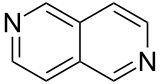 | naphthyridine | 253-50-9 |
References
- Desmond J. Brown, Jonathan A. Ellman, Edward C. Taylor: The Chemistry of Heterocyclic Compounds, The Naphthyridines, John Wiley & Sons, 2007.
- Paudler, William W.; Kress, Thomas J. (1968). "Naphthyridine chemistry. IX. Bromination and animation of the 1,X-naphthyridines". The Journal of Organic Chemistry. 33 (4): 1384–1387. doi:10.1021/jo01268a018.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.