Dibutylone
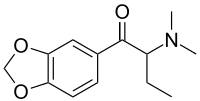
Dibutylone (bk-DMBDB[2]) is a stimulant drug of the amphetamine, phenethylamine, and cathinone drug classes. It is structurally related to butylone, a designer drug that has been detected in products marketed as bath salts or plant food.[3]
 | |
| Names | |
|---|---|
| Systematic IUPAC name
1-(Benzo[d][1,3]dioxol-5-yl)-2-(dimethylamino)butan-1-one | |
| Other names
1-(1,3-Benzodioxol-5-yl)-2-(dimethylamino)butan-1-one; β-Keto-dimethylbenzodioxolylbutanamine; bk-DMBDB | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C13H17NO3 | |
| Molar mass | 235.283 g·mol−1 |
| Pharmacology | |
| Legal status |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
In 2018, dibutylone was the third most common drug of the cathinone class to be identified in Drug Enforcement Administration seizures.[4]
Legal status
In United States, dibutylone is on the list of Schedule I Controlled Substances as a positional isomer of pentylone.[5]
References
- Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- "Southern Association of Forensic Scientists". Archived from the original on 2013-03-25. Retrieved 2012-12-12.
- Krotulski, Alex J; Mohr, Amanda L A; Papsun, Donna M; Logan, Barry K (2018). "Dibutylone (bk-DMBDB): Intoxications, Quantitative Confirmations and Metabolism in Authentic Biological Specimens". Journal of Analytical Toxicology. 42 (7): 437–445. doi:10.1093/jat/bky022. PMID 29554274.
- "Emerging Threat Report: Annual 2018" (PDF). Special Testing and Research Laboratory, Drug Enforcement Administration.
- "Controlled Substances" (PDF). Drug Enforcement Administration.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.