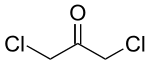
Bis(chloromethyl) ketone
Bis(chloromethyl) ketone is a chemical substance with formula C
3H
4Cl
2O. It is a solid, and is used in the making of citric acid. Exposures such as contact or inhalation of bis(chloromethyl) ketone can result in irritation or damage to skin, eyes, throat, lungs, liver and kidneys, as well as headaches and fainting.[1]
 | |||
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,3-Dichloropropan-2-one | |||
| Other names
1,3-Dichloroacetone α,α'-Dichloroacetone | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.806 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
| UN number | 2649 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C3H4Cl2O | |||
| Molar mass | 126.96 g·mol−1 | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
Extremely toxic. Dangerous to the skin and eyes | ||
| GHS labelling: | |||
    | |||
| Danger | |||
| H300, H310, H314, H330, H341, H410 | |||
| P201, P202, P260, P262, P264, P270, P271, P273, P280, P281, P284, P301+P310, P301+P330+P331, P302+P350, P303+P361+P353, P304+P340, P305+P351+P338, P308+P313, P310, P320, P321, P322, P330, P361, P363, P391, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Legal aspects
Bis(chloromethyl) ketone is a substance which is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[2]
See also
References
- Hazardous Substance Fact Sheet from the New Jersey Department of Health and Senior Services
- 40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities (PDF) (Report) (July 1, 2008 ed.). Government Printing Office. Archived from the original (PDF) on February 25, 2012. Retrieved October 29, 2011.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.


