Dichloromethyl methyl ether
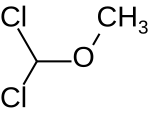
Dichloromethyl methyl ether (HCl2COCH3) is an organic compound that belongs to the class of ethers with a dichloromethyl group and a methyl group. It can be synthesized from methyl formate and a mixture of phosphorus pentachloride and phosphorus oxychloride[1] or by chlorination of chlorodimethyl ether.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Dichloro(methoxy)methane | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.023.180 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H4Cl2O | |
| Molar mass | 114.95 g·mol−1 |
| Boiling point | 85°C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The compound is used in the formylation of aromatic compounds (Rieche formylation) and as a chlorination agent in the formation of acid chlorides.[2]
References
- Organic Syntheses, Coll. Vol. 5, p.365 (1973); Vol. 47, p.51 (1967). link
- Organic Syntheses, Coll. Vol. 7, p.467 (1990); Vol. 61, p.1 (1983). Link
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.