Dicyclopentadiene
Dicyclopentadiene, abbreviated DCPD, is a chemical compound with formula C10H12. At room temperature, it is a white brittle wax, although lower purity samples can be straw coloured liquids. The pure material smells somewhat of soy wax or camphor, with less pure samples possessing a stronger acrid odor. Its energy density is 10,975 Wh/l. Dicyclopentadiene is a co-produced in large quantities in the steam cracking of naphtha and gas oils to ethylene. The major use is in resins, particularly, unsaturated polyester resins. It is also used in inks, adhesives, and paints.
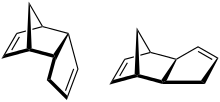 endo‑Dicyclopentadiene (left) exo‑Dicyclopentadiene (right) | |
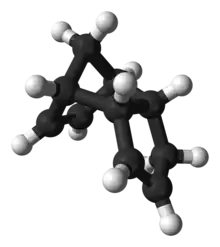 Ball-and-stick model of endo‑Dicyclopentadiene | |
| Names | |
|---|---|
| IUPAC name
Tricyclo[5.2.1.02,6]deca-3,8-diene | |
| Other names
1,3-Dicyclopentadiene, Bicyclopentadiene, 3a,4,7,7a-Tetrahydro-1H-4,7-methanoindene
| |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | DCPD |
| 1904092 | |
| ChemSpider | |
| ECHA InfoCard | 100.000.958 |
| EC Number |
|
| KEGG | |
| MeSH | Dicyclopentadiene |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | UN 2048 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H12 | |
| Molar mass | 132.20 g/mol |
| Appearance | Colorless, crystalline solid[2] |
| Odor | camphor-like[2] |
| Density | 0.978 g/cm3 |
| Melting point | 32.5 °C (90.5 °F; 305.6 K) |
| Boiling point | 170 °C (338 °F; 443 K) |
| 0.02%[2] | |
| Solubility | very soluble in ethyl ether, ethanol soluble in acetone, dichloromethane, ethyl acetate, n-hexane, toluene |
| log P | 2.78 |
| Vapor pressure | 180 Pa (20 °C)[2] |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 32 °C (90 °F; 305 K) |
| 503 °C (937 °F; 776 K) | |
| Explosive limits | 0.8%-6.3%[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
none[2] |
REL (Recommended) |
TWA 5 ppm (30 mg/m3)[2] |
IDLH (Immediate danger) |
N.D.[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The top seven suppliers worldwide together had an annual capacity in 2001 of 179 kilotonnes (395 million pounds).
DPCD was discovered as a C10H12 hydrocarbon among the products of pyrolysis of phenol by Henry Roscoe, who didn't identify the structure (that was made during the following decade) but accurately assumed that it was a dimer of some C5H6 hydrocarbon.[3]
History and structure
For many years the structure of dicyclopentadiene was thought to feature a cyclobutane ring as the fusion between the two subunits. Through the efforts of Alder and coworker, the structure was deduced in 1931.[4]
The spontaneous dimerization of neat cyclopentadiene at room temperature to form dicyclopentadiene proceeds to around 50% conversion over 24 hours and yields the endo isomer in better than 99:1 ratio as the kinetically favored product (about 150:1 endo:exo at 80 °C).[5] However, prolonged heating results in isomerization to the exo isomer. The pure exo isomer was first prepared by base-mediated elimination of hydroiodo-exo-dicyclopentadiene.[6] Thermodynamically, the exo isomer is about 0.7 kcal/mol more stable than the endo isomer.[7] The exo isomer also has a lower reported melting point of 19°C.[8] Both isomers are chiral.

Reactions
Above 150 °C, dicyclopentadiene undergoes a retro-Diels–Alder reaction at an appreciable rate to yield cyclopentadiene. The reaction is reversible and at room temperature cyclopentadiene dimerizes over the course of hours to re-form dicyclopentadiene. Cyclopentadiene is a useful diene in Diels–Alder reactions as well as a precursor to metallocenes in organometallic chemistry. It is not available commercially as the monomer, due to the rapid formation of dicyclopentadiene; hence, it must be prepared by "cracking" the dicyclopentadiene (heating the dimer and isolating the monomer by distillation) shortly before it is needed.
The thermodynamic parameters of this process have been measured. At temperatures above about 125 °C in the vapor phase, dissociation to cyclopentadiene monomer starts to become thermodynamically favored (the dissociation constant Kd = [cyclopentadiene]2 / [dicyclopentadiene] > 1). For instance, the values of Kd at 149 °C and 195 °C were found to be 277 and 2200, respectively.[9] By extrapolation, Kd is on the order of 10–4 at 25 °C, and dissociation is disfavored. In accord with the negative values of ΔH° and ΔS° for the Diels–Alder reaction, dissociation of dicyclopentadiene is more thermodynamically favorable at high temperatures. Equilibrium constant measurements imply that ΔH° = –18 kcal/mol and ΔS° = –40 eu for cyclopentadiene dimerization.[10]
Dicyclopentadiene polymerizes. Copolymers are formed with ethylene or styrene. The "norbornene double bond" participates.[11] Using ring-opening metathesis polymerization a homopolymer polydicyclopentadiene is formed.
Hydroformylation of DCP gives the dialdehyde called TCD dialdehyde (TCD = tricyclodecane). This dialdehyde can be oxidized to the dicarboxylic acid and to a diol. All of these derivatives have some use in polymer science.[12]
Hydrogenation of dicyclopentadiene gives tetrahydrodicyclopentadiene (C
10H
16), which is a component of jet fuel JP-10,[13] and rearranges to adamantane[14][15] with aluminium chloride or acid at elevated temperature.

References
- Merck Index, 11th Edition, 2744
- NIOSH Pocket Guide to Chemical Hazards. "#0204". National Institute for Occupational Safety and Health (NIOSH).
- Levandowski, B. J.; Raines, R. T. (2021). "Click Chemistry with Cyclopentadiene". Chemical Reviews. 121 (12): 6777–6801. doi:10.1021/acs.chemrev.0c01055. PMC 8222071. PMID 33651602.
- Roberts, John D.; Sharts, Clay M. (2011). "Cyclobutane Derivatives from Thermal Cycloaddition Reactions". Organic Reactions. pp. 1–56. doi:10.1002/0471264180.or012.01. ISBN 978-0471264187.
- Xu, Rui; Jocz, Jennifer N.; Wiest, Lisa K.; Sarngadharan, Sarath C.; Milina, Maria; Coleman, John S.; Iaccino, Larry L.; Pollet, Pamela; Sievers, Carsten; Liotta, Charles L. (2019-09-05). "Cyclopentadiene Dimerization Kinetics in the Presence of C5 Alkenes and Alkadienes". Industrial & Engineering Chemistry Research. 58 (50): 22516–22525. doi:10.1021/acs.iecr.9b04018. ISSN 0888-5885. S2CID 202876152.
- Bartlett, Paul D.; Goldstein, Irving S. (1947-10-01). "exo-Dicyclopentadiene". Journal of the American Chemical Society. 69 (10): 2553. doi:10.1021/ja01202a501. ISSN 0002-7863.
- Narayan, Adithyaram; Wang, Beibei; Nava Medina, Ilse Belen; Mannan, M. Sam; Cheng, Zhengdong; Wang, Qingsheng (2016-11-01). "Prediction of heat of formation for exo-Dicyclopentadiene". Journal of Loss Prevention in the Process Industries. 44: 433–439. doi:10.1016/j.jlp.2016.10.015. ISSN 0950-4230.
- Jamróz, Małgorzata E; Gałka, Sławomir; Dobrowolski, Jan Cz (September 2003). "On dicyclopentadiene isomers". Journal of Molecular Structure: THEOCHEM. 634 (1–3): 225–233. doi:10.1016/S0166-1280(03)00348-8.
- Wilson, Philip J.; Wells, Joseph H. (1944-02-01). "The Chemistry and Utilization of Cyclopentadiene". Chemical Reviews. 34 (1): 1–50. doi:10.1021/cr60107a001. ISSN 0009-2665.
- Lenz, Terry G.; Vaughan, John D. (1989-02-01). "Employing force-field calculations to predict equilibrium constants and other thermodynamic properties for the dimerization of 1,3-cyclopentadiene". The Journal of Physical Chemistry. 93 (4): 1592–1596. doi:10.1021/j100341a081. ISSN 0022-3654.
- Li, Xiaofang; Hou, Zhaomin (2005). "Scandium-Catalyzed Copolymerization of Ethylene with Dicyclopentadiene and Terpolymerization of Ethylene, Dicyclopentadiene, and Styrene". Macromolecules. 38 (16): 6767. Bibcode:2005MaMol..38.6767L. doi:10.1021/ma051323o.
- Kohlpaintner, Christian; Schulte, Markus; Falbe, Jürgen; Lappe, Peter; Weber, Jürgen (2008). "Aldehydes, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_321.pub2.
- "Combustion Chemistry". Department of Chemistry, College of Science, the University of Utah. The University of Utah. Retrieved 12 January 2022.
- Schleyer, Paul von R.; Donaldson, M. M.; Nicholas, R. D.; Cupas, C. (1973). "Adamantane". Organic Syntheses.; Collective Volume, vol. 5, p. 16
- Hönicke, Dieter; Födisch, Ringo; Claus, Peter; Olson, Michael (2002). "Cyclopentadiene and Cyclopentene". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_227.
External links
- MSDS for dicyclopentadiene
- Inchem fact sheet for dicyclopentadiene
- CDC — NIOSH Pocket Guide to Chemical Hazards
