Dienochlor
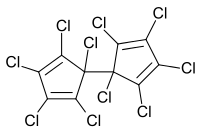
Dienochlor is an organochlorine compound included in the group of cyclic chlorinated hydrocarbons. Its chemical formula is C
10Cl
10.[1] Dienochlor is mostly used as a pesticide and ovicide.
 | |
| Names | |
|---|---|
| IUPAC name
1,2,3,4,5-Pentachloro-5-(1,2,3,4,5-pentachlorocyclopenta-2,4-dien-1-yl)cyclopenta-1,3-diene | |
| Other names
Decachlor, perchlorbis(cyclopenta-2,4-dien-1-yl), pentac | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.017.058 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10Cl10 | |
| Molar mass | 474.61 g·mol−1 |
| Appearance | Yellow crystalline solid |
| Density | 1.923 g/cm3 |
| Melting point | 122–123 °C (252–253 °F; 395–396 K) |
| Boiling point | 250 °C (482 °F; 523 K) |
| Practically insoluble in water | |
| Hazards | |
| GHS labelling: | |
  | |
| Warning | |
| H302, H319, H400 | |
| P264, P270, P273, P280, P301+P312, P305+P351+P338, P313, P330, P337, P391, P501 | |
| Flash point | 187.8 °C (370.0 °F; 460.9 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
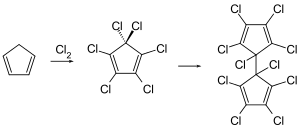
Dienochlor can be obtained by catalytic reduction of hexachlorocyclopentadiene (e.g. with copper or hydrogen).[2]
Properties
Dienochlor is a combustible yellow solid which is practically insoluble in water. It decomposes when heated above 250 °C. It decomposes rapidly under the influence of sunlight.
Applications
Dienochlor is used as an acaricide under the trade name Pentac for combating mites (Tetranychus, Polyphagotarsonemus latus) on roses, chrysanthemums, and other ornamental plants.[3]
Regulations
Dienochlor was approved for use in the Western Germany between 1971 and 1990. In the European Union, no plant protection products containing dienochlor are authorized.[4]
References
- "Dienochlor". Retrieved 1 June 2017.
- "Dienochlor PESTANAL®, analytical standard". Sigma Aldrich. sigmaaldrich.com.
- "PENTAC". toxnet.nlm.nih.gov. Retrieved 1 June 2017.
- "VERORDNUNG (EG) Nr. 2076/2002 DER KOMMISSION". eur-lex.europa.eu. Retrieved 1 June 2017.