Captafol
Captafol is a fungicide. It is used to control almost all fungal diseases of plants except powdery mildews.[2] It is believed to be a human carcinogen, and production for use as a fungicide in the United States stopped in 1987. Its continued use from existing stocks was allowed, but in 1999 the Environmental Protection Agency banned its use on all crops except onions, potatoes, and tomatoes. In 2006 even these exceptions were disallowed, so currently its use on all crops is banned in the United States. Several other countries have followed suit since 2000, and as of 2010, no countries are known to allow the use of captafol on food crops.[3] Currently, the National Institute for Occupational Safety and Health established a recommended exposure limit of 0.1 mg/m3 for dermal exposures.[4]
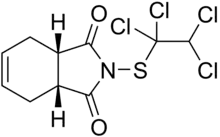 | |
| Names | |
|---|---|
| Preferred IUPAC name
(3aR,7aS)-2-[(1,1,2,2-Tetrachloroethyl)sulfanyl]-3a,4,7,7a-tetrahydro-1H-isoindole-1,3(2H)-dione | |
| Other names
cis-Captafol; Merpafol; Crisfolatan; Sulfonimide; Sulpheimide; Arborseal; Captaspor; Mycodifol; Pillartan; Terrazol; Difolatan | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.017.604 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H9Cl4NO2S | |
| Molar mass | 349.05 g·mol−1 |
| Appearance | White, crystalline solid |
| Melting point | 161 °C; 321 °F; 434 K[1] |
| Boiling point | decomposes |
| 0.0001%[1] | |
| Vapor pressure | 0.000008 mmHg (20°C) [1] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
potential occupational carcinogen |
| Flash point | noncombustible[1] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
none[1] |
REL (Recommended) |
Ca TWA 0.1 mg/m3 [skin][1] |
IDLH (Immediate danger) |
N.D.[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Captafol was disclosed in US patent 3,178,447 (1965).[5] Its synergistic mixture with thiabendazole was described in US patent 4092422 (1978).[6]
International trade in captafol is regulated by the Rotterdam Convention.
References
- NIOSH Pocket Guide to Chemical Hazards. "#0098". National Institute for Occupational Safety and Health (NIOSH).
- Captafol from Extension Toxicology Network
- Captafol CAS No. 2425-06-1 Reasonably anticipated to be a human carcinogen National Institute of Health, Report on Carcinogens, Twelfth Edition (2011)
- NIOSH Pocket Guide to Chemical Hazards from Centers of Disease Control and Prevention
- "N-polyhaloalkylthio compounds". 1965-04-13. US patent 3,178,447.. The term "captafol" is not used in this publication, but is described as disclosed in this patent in patent application 20080269051
- "Synergistic fungicidal mixture of captafol and thiabendazol [sic]". US patent via PatentLens.
Further reading
- Captafol in the Pesticide Properties DataBase (PPDB)