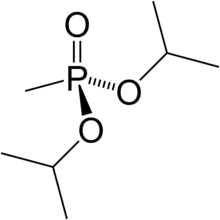
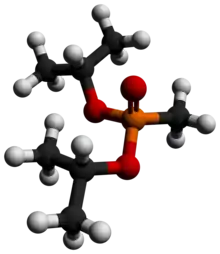
Diisopropyl methylphosphonate
Diisopropyl methylphosphonate (DIMP), also known as diisopropyl methane-phosphonate and phosphonic acid and methyl-bis-(1-methylethyl)ester, is a chemical by-product in the production of sarin gas.[1]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Di(propan-2-yl) methylphosphonate | |
| Other names
2-(Methyl-propan-2-yloxyphosphoryl)oxypropane | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | DIMP |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.014.451 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H17O3P | |
| Molar mass | 180.184 g·mol−1 |
| Density | 0.976 g/mL |
| Boiling point | 215 °C (419 °F; 488 K) |
| Hazards | |
| Flash point | 98 °C (208 °F; 371 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
DIMP is a colorless liquid that has been shown to affect the hematological (blood forming) system in animals.[2] Its chemical formula is C7H17O3P.[3]
History
DIMP is a chemical by-product resulted from the manufacture of sarin (GB).[4]
Use
No commercial uses of DIMP are known to exist.[5]
Occurrences
DIMP is not known to occur naturally in the environment.
Productions
Synthesis
DIMP can be prepared by a gradual addition of triisopropyl phosphite with methyl iodide, utilizing distillation technique.
References
- "ATSDR - Toxic Substances - Diisopropyl Methylphosphonate (DIMP)". Atsdr.cdc.gov. 2011-03-03. Retrieved 2012-10-18.
- "tf119" (PDF). Retrieved 2012-10-18.
- "Center of Chemicals". Chemicals.pl. Retrieved 2012-10-18.
- ATSDR – Toxic Substances – Diisopropyl Methylphosphonate (DIMP)
- http://www.atsdr.cdc.gov/toxprofiles/tp119-c4.pdf
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.