Dimethylbenzylamine
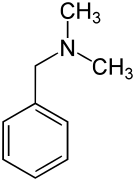
Dimethylbenzylamine is the organic compound with the formula C6H5CH2N(CH3)2. The molecule consists of a benzyl group, C6H5CH2, attached to a dimethylamino functional group. It is a colorless liquid. It is used as a catalyst for the formation of polyurethane foams and epoxy resins.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
N,N-Dimethyl-1-phenylmethanamine | |||
| Other names
N,N-Dimethylbenzenemethanamine, N,N-Dimethylbenzylamine, N-Benzyldimethylamine, Dimethylbenzylamine, Benzyl-N,N-dimethylamine, N-(Phenylmethyl)dimethylamine, BDMA, Sumine 2015, Benzenemethanamine, Dabco B-16, Araldite accelerator 062, N,N-Dimethyl(phenyl)methanamine, DMBA[1] | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.863 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2619 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C9H13N | |||
| Molar mass | 135.210 g·mol−1 | ||
| Appearance | colourless liquid | ||
| Density | 0.91 g/cm3 at 20 °C | ||
| Melting point | −75 °C (−103 °F; 198 K) | ||
| Boiling point | 180 to 183 °C (356 to 361 °F; 453 to 456 K) | ||
| 1.2 g/100mL | |||
| Hazards | |||
| GHS labelling: | |||
   | |||
| Danger | |||
| H226, H302, H312, H314, H332, H412 | |||
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P273, P280, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P322, P330, P363, P370+P378, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 55 °C (131 °F; 328 K) | ||
| 410 °C (770 °F; 683 K) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Synthesis
N,N-Dimethylbenzylamine can be synthesized by the Eschweiler–Clarke reaction of benzylamine[2][3]
Reactions
It undergoes directed ortho metalation with butyl lithium:
- [C6H5CH2N(CH3)2 + BuLi → 2-LiC6H4CH2N(CH3)2
- LiC6H4CH2N(CH3)2 + E+ → 2-EC6H4CH2N(CH3)2
Via these reactions, many derivatives are known with the formula 2-X-C6H4CH2N(CH3)2 (E = SR, PR2, etc.).
The amine is basic and undergoes quaternization with alkyl halides (e.g. hexyl bromide) to give quaternary ammonium salts:[4]
- [C6H5CH2N(CH3)2 + RX → [C6H5CH2N(CH3)2R]+X−
Such salts are useful phase transfer catalysts.
Uses
As the molecule has tertiary amine functionality, two of the key uses are as an epoxy-amine cure enhancement catalyst and also as a polyurethane catalyst.[5][6][7][8]
References
- "N,N-dimethyl benzyl amine". The Good Scents Company. Retrieved 1 November 2020.
- Icke, R. N.; Wisegarver, B. B.; Alles, G. A. (1945). "β-Phenylethyldimethylamine". Organic Syntheses. 25: 89. doi:10.15227/orgsyn.025.0089.
- Clarke, H. T.; Gillespie, H. B.; Weisshaus, S. Z. (1933). "The Action of Formaldehyde on Amines and Amino Acids". J. Am. Chem. Soc. 55 (11): 4571. doi:10.1021/ja01338a041.
- W. R. Brasen; C. R. Hauser (1954). "o-Methylethylbenzyl Alcohol". Org. Synth. 34: 58. doi:10.15227/orgsyn.034.0058.
- Firouzmanesh, Mr; Azar, A Aref (June 2003). "Study of the effect of BDMA catalyst in the epoxy novolac curing process by isothermal DSC". Polymer International. 52 (6): 932–937. doi:10.1002/pi.1135. ISSN 0959-8103.
- Firouzmanesh, M. R.; Azar, A. Aref (March 2005). "Study of the Effect of BDMA Catalyst in Epoxy Novolac Curing Process by Isothermal DSC". Journal of Reinforced Plastics and Composites. 24 (4): 345–353. doi:10.1177/0731684405033953. ISSN 0731-6844.
- Zhang, Qian; Hu, Xiang-Ming; Wu, Ming-Yue; Zhao, Yan-Yun; Yu, Chuang (2018-07-15). "Effects of different catalysts on the structure and properties of polyurethane/water glass grouting materials". Journal of Applied Polymer Science. 135 (27): 46460. doi:10.1002/app.46460.
- Mascorro, José A. (2003). "Benzyldimethylamine (BDMA): Catalyst of Choice with Epoxy Embedding Media". Microscopy Today. 11 (4): 47. doi:10.1017/s1551929500053104. Retrieved 2023-07-19.