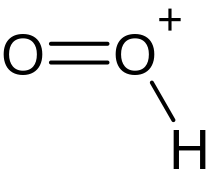
Dioxidanylium
Dioxidanylium, which is protonated molecular oxygen, or just protonated oxygen, is an ion with formula HO+
2.
It is formed when hydrogen containing substances combust, and exists in the ionosphere, and in plasmas that contain oxygen and hydrogen.[2] Oxidation by O2 in superacids could be by way of the production of protonated molecular oxygen.
 | |
| Names | |
|---|---|
| IUPAC name
oxooxidanium | |
| Other names
Hydroperoxy cation; Hydridodioxygen(1+); Dioxidenium; dioxidanylium | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| 508 | |
PubChem CID |
|
| |
| |
| Properties | |
| HO2+ | |
| Molar mass | 33.005 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
It is the conjugate acid of dioxygen. The proton affinity of dioxygen (O2) is 4.4 eV.[3]
Significance
Protonated molecular oxygen is of interest in trying to detect dioxygen in space. Because Earth's atmosphere is full of O2, its spectrum from a space object is impossible to observe from the ground. However HO+
2 should be much more detectable.[4]
Formation
Reaction of dioxygenyl O+
2 with hydrogen:[5]
- O+•
2 + H2 → HO+
2 + H•
The reaction of the trihydrogen cation with dioxygen is approximately thermoneutral:[3]
- O2 + H+
3 → HO+
2 + H2
When atomic hydrogen, created in an electric discharge is rapidly cooled with oxygen and condensed in solid neon, several reactive ions and molecules are produced. These include HO2 (hydroperoxyl), HOHOH−, H2O(HO), HOHO− as well as HO+
2.[6] This reaction also forms hydrogen peroxide (H2O2) and hydrogen tetroxide (H2O4).[7]
Properties
In the infrared spectrum HO+
2 the v1 band due to vibrating O–H has a band head at 3016.73 cm−1.[8]
Reactions
A helium complex (He–O2H+) also is known.[8]
HO+
2 appears to react rapidly with hydrogen:[9]
- HO+
2 + H2 → O2 + H+
3
HO+
2 also reacts with dinitrogen and water:[9]
- HO+
2 + H2O → O2 + H3O+
Related
The protonated molecular oxygen dimer HO+
4 has a lower energy than that of protonated molecular oxygen.[3]
References
- "HO2+". webbook.nist.gov.
- Robbe, J.M.; Monnerville, M.; Chambaud, G.; Rosmus, P.; Knowles, P.J. (January 2000). "Theoretical spectroscopic data of the HO+
2 ion". Chemical Physics. 252 (1–2): 9–16. Bibcode:2000CP....252....9R. doi:10.1016/S0301-0104(99)00350-X. - Xavier, George D.; Bernal-Uruchurtu, Margarita I.; Hernández-Lamoneda, Ramón (28 August 2014). "Communication: study of O4H+: A tracer molecule in the interstellar medium?". The Journal of Chemical Physics. 141 (8): 081101. doi:10.1063/1.4894068. PMID 25172995.
- Widicus Weaver, Susanna L.; Woon, David E.; Ruscic, Branko; McCall, Benjamin J. (20 May 2009). "Is HO+
2 a Detectable Interstellar Molecule?". The Astrophysical Journal. 697 (1): 601–609. Bibcode:2009ApJ...697..601W. doi:10.1088/0004-637X/697/1/601. - Ajello, J. M. (1974). "Formation of HO+
2 by reaction of metastable O+
2 ions with H2". The Journal of Chemical Physics. 60 (4): 1211–1213. Bibcode:1974JChPh..60.1211A. doi:10.1063/1.1681184. - Jacox, Marilyn E.; Thompson, Warren E. (24 December 2012). "Infrared Spectra of Products of the Reaction of H Atoms with O2 Trapped in Solid Neon: HO2, HO+
2, HOHOH− , and H2O(HO)". The Journal of Physical Chemistry A. 117 (39): 9380–9390. doi:10.1021/jp310849s. PMID 23215001. - Levanov, A. V.; Isaikina, O. Ya.; Antipenko, E. E.; Lunin, V. V. (5 August 2014). "Mechanism of the formation of hydrogen tetroxide and peroxide via low-temperature interaction between hydrogen atoms and molecular oxygen". Russian Journal of Physical Chemistry A. 88 (9): 1488–1492. Bibcode:2014RJPCA..88.1488L. doi:10.1134/S0036024414090222. S2CID 97672680.
- Kohguchi, Hiroshi; Jusko, Pavol; Yamada, Koichi M. T.; Schlemmer, Stephan; Asvany, Oskar (14 April 2018). "High-resolution infrared spectroscopy of O2H+ in a cryogenic ion trap". The Journal of Chemical Physics. 148 (14): 144303. Bibcode:2018JChPh.148n4303K. doi:10.1063/1.5023633. PMID 29655341.
- Kluge, Lars; Gärtner, Sabrina; Brünken, Sandra; Asvany, Oskar; Gerlich, Dieter; Schlemmer, Stephan (13 November 2012). "Transfer of a proton between H2 and O2". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 370 (1978): 5041–5054. Bibcode:2012RSPTA.370.5041K. doi:10.1098/rsta.2012.0170. PMID 23028152.