Diphosphorus trisulfide
Diphosphorus trisulfide (sometimes called phosphorus trisulfide) is a phosphorus sulfide with the formula of P2S3. The substance is highly unstable and difficult to study.[3]
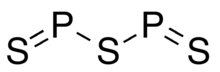 | |
| Names | |
|---|---|
| IUPAC name
Phosphorus trisulfide | |
| Systematic IUPAC name
Diphosphorus trisulfide | |
| Other names
Diphosphathiane-1,3-dithione Phosphorus(III) sulfide[1] | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| P2S3 | |
| Molar mass | 158.13 g·mol−1 |
| Appearance | Grayish yellow solid[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
History
Early reports that diphosphorous trisulfide could be formed by heating red phosphorus and sulfur were shown to be incorrect by Helff in 1893. Its existence was again reported by Ralston and Wilkinson in 1928. In 1959, Pitochelli and Audrieth showed that the substance existed by X-ray diffraction but did not succeed in fully isolating it.[4] In 1997, Lohr and Sundholm published a theoretical analysis of the potential structures of this molecular substance.[5]
In 2017, Xiao proposed that a 2D crystallisation of P2S3 was possible based on computer simulations. Xiao suggested that nanoribbons and nanotubes of the material may have applications in semiconductor electronics.[6]
Properties
P2S3 is highly flammable. The solid may spontaneously ignite with moist air or in contact with water. Produces phosphoric acid and hydrogen sulfide, a toxic flammable gas in reaction with water. P2S3 is a strong reducing agent. Reacts vigorously with oxidizing agents, including inorganic oxoacids, organic peroxides and epoxides. Produce acidic and corrosive phosphorus pentoxide and sulfur dioxide when burned.[7]
References
- "Phosphorus sulfide (P2S3)".
- "Phosphorus sulfide (P2S3)".
- Pitochelli & Audrieth, p. 4458
- Pitochelli & Audrieth, p. 4458
- Lohr & Sundholm, p. 495
- Xiao, pp. 6–7
- "Phosphorus sulfide (P2S3)".
Bibliography
- Lohr, Lawrence L.; Sundholm, Dage, "An ab initio characterization of diphosphorus trisulfide, P2S3", Journal of Molecular Structure, vol. 413–414, pp. 495–500, 30 September 1997. doi:10.1016/S0022-2860(97)00142-7
- Pitochelli, A.R.; Audrieth, L.F., "Concerning the existence of diphosphorus trisulfide", Journal of the American Chemical Society, vol. 81, iss. 17, pp. 4458–4460, 1 September 1959. doi:10.1021/ja01526a005
- Ralston, A.W.; Wilkinson, J.A., "Reactions in liquid hydrogen sulfide. III thiohydrolysis of chlorides", Journal of the American Chemical Society, vol. 50, iss. 2, pp. 258–264, 1 February 1928. doi:10.1021/ja01389a002
- Xiao, Hang, Low-Dimensional Material: Structure-Property Relationship and Applications in Energy and Environmental Engineering (PhD Dissertation), Columbia University ProQuest Dissertations Publishing, no. 10615524, 2017.