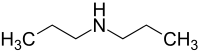
Dipropylamine
Dipropylamine is a flammable, highly toxic, corrosive amine. It occurs naturally in tobacco leaves and artificially in industrial wastes.[3] Exposure can cause excitement followed by depression, internal bleeding, dystrophy, and severe irritation.[2]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N-Propylpropan-1-amine | |
| Other names
(Dipropyl)amine | |
| Identifiers | |
3D model (JSmol) |
|
| 505974 | |
| ChemSpider | |
| ECHA InfoCard | 100.005.060 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2383 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties[1] | |
| C6H15N | |
| Molar mass | 101.193 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Ichtyal, ammoniacal |
| Density | 738 mg mL−1 |
| Melting point | −63.00 °C; −81.40 °F; 210.15 K |
| Boiling point | 109 to 111 °C; 228 to 232 °F; 382 to 384 K |
| Solubility in diethyl ether | Miscible |
Henry's law constant (kH) |
190 μmol Pa−1 kg−1 |
Refractive index (nD) |
1.4049 |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
−156.1–−153.1 kJ mol−1 |
Std enthalpy of combustion (ΔcH⦵298) |
−4.3515–−4.3489 MJ mol−1 |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H225, H302, H312, H314, H332 | |
| P210, P280, P305+P351+P338, P310 | |
| Flash point | 7 °C (45 °F; 280 K) |
| 280 °C (536 °F; 553 K) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
200–400 mg kg−1 (rat)[2] |
| Related compounds | |
Related amines |
|
Related compounds |
Agmatine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Lide, D. R. (1998). Handbook of Chemistry and Physics (87th ed.). Boca Raton, Florida: CRC Press. p. 447. ISBN 0-8493-0594-2.
- Grushko, Ya. M. (1992). Kotlobye, A. P. (ed.). Handbook of Dangerous Properties of Inorganic and Organic Substances in Industrial Wastes. Boca Raton: CRC Press. p. 232. ISBN 0-8493-9300-0. Retrieved 2009-04-07.
- Howard, P. H., ed. (2003). Fate and Exposure Data for Organic Compounds. Vol. 5. Boca Raton, Florida: CRC Press. pp. 177–180. ISBN 0-87371-976-X. Retrieved 2009-04-07.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.