Dithiin
Dithiin /daɪ.ˈθaɪ.ɪn/ is a class of heterocyclic compounds, with the parent members having the formula (C2H2)2S2. Two isomers of this parent are recognized, 1,2- and 1,4-dithiins. Planar dithiins are 8π e− systems, which would lead to antiaromaticity if the structure was planar. Akin to the behavior of cyclooctatetraene, they instead adopt nonplanar structures. Vinyldithiin, a common component of garlic, is a misnomer for 3-vinyl-4H-1,2-dithiin. 1,3-dithiins are unknown.
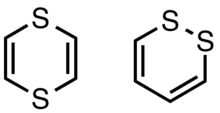
1,4-Dithiins
1,4-Dithiins have been more extensively studied. They are usually prepared by condensation of the equivalent of α-mercaptocarbonyls. For example, the acetal HSCH2CH(OEt)2 converts upon heating to the parent 1,4-dithiin. They are nonplanar and can be oxidized to their radical cations. Photolysis leads to dimerization via a [2+2] cycloaddition.[1] Thianthrene is dibenzo-1,4-dithiin.
1,2-Dithiins
1,2-Dithiins are isomers of but-2-ene-dithials. They tend to be unstable with respect to loss of sulfur and formation of the thiophene derivative:[2]
- C4R4S2 → C4R4S + "S"
They are often claret-colored. Some occur as flower pigments in plants of the family Asteraceae.[3]
References
- Keiji Kobayashia & Chhabi L. Gajurela "The Chemistry of 1, 4-Dithiins" Sulfur Reports, 1986, Volume 7, pp. 123-148. doi:10.1080/17415998609410046
- Block, Eric et al, "Synthesis, Properties, Oxidation, and Electrochemistry of 1, 2-Dichalcogenins" Journal of the American Chemical Society (2000), 122(21), 5052-5064. doi:10.1021/ja994134s
- Block, Eric; Page, Jon; Toscano, John P.; Wang, Cun-Xiao; Zhang, Xing; DeOrazio, Russell; Guo, Chuangxing; Sheridan, Robert S.; Towers, G. H. Neil, "Photochemistry of Thiarubrine A and Other 1, 2-Dithiins: Formation of 2, 6-Dithiabicyclo[3.1.0]hex-3-enes" Journal of the American Chemical Society 1996, vol. 118, pp. 4719-20. doi:10.1021/ja960589v