IGSF6
IGSF6 is a protein that in humans is encoded by the IGSF6 gene.[5][6]
| IGSF6 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | IGSF6, DORA, immunoglobulin superfamily member 6 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 606222 MGI: 1891393 HomoloGene: 36189 GeneCards: IGSF6 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Overview
In humans, the immunoglobulin superfamily member 6 (IGSF6) gene with alias DORA encodes CD8 protein IGSF6 (24 kDA) with orthologs in mammals, birds, reptiles, and bony fishes.[7] IGSF6 is located on the complement strand of chromosome 16 (16p12.2) spanning 13059 base pairs and is located entirely within an intron of the gene METTL9.[8] IGSF6 is predicted to be an integral component of the plasma membrane and contribute to immune response.[9] It is also predicted to be involved in cell surface receptor signaling and enable transmembrane signaling receptor activity. IGSF6 gene was localized to a locus associated with inflammatory bowel disease (IBD). However, there was no association with single nucleotide polymorphisms (SNPs) and IBD in patients with the disease.[10]
Gene
A common alias for IGSF6 is downregulated by activation (DORA). The cytogenic location is on chromosome 16 (16p12.2). IGSF6 has 6 exons total.[11] The span of the gene is 13059 base pairs. [12]
Proteins
The theoretical isoelectric point (pI) and molecular weight (mw) for the IGSF6 protein are 8.9 and 27 kDa, respectively, before any modification. The pI of the protein is not consistent throughout, as the N-terminal half has a lower pI than the C-terminal half. IGSF6 is neutral at 8.93 and would be negative around 7.[13]
Eukaryotic Linear Motif (ELM) was used to find protein Motifs. The list ELM provided was after globular domain filtering, structural filtering, and context filtering. The four Motifs shown are organized by probability and are conserved in mammalian orthologs.[14]

Structure
The secondary structure of IGSF6 is predicted to have regions of coils, strands, and alpha helices.[15] The most pronounced helix regions occur from amino acids 149-178 and amino acids 197-218. [16][17][18][19]
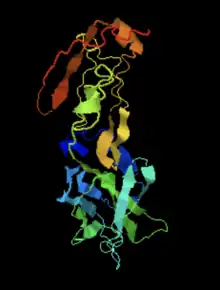
IGSF6 contains a transmembrane domain from amino acids 154 to 176.[20] The predicted disulfide bonds were found using DiANNA. [21]

Gene Level Regulation
Expression Pattern
IGSF6 is highly expressed in white blood cells and secondary lymphoid organs including the lymph nodes and spleen.[22] The mRNA abundance across 20 human tissues is low.[23] The micro-array assessed tissue expression patterns showed high expression in ganglia, monocytes, and myeloid tissue.[24] In situ hybridization showed that the regulation of IGSF6 was low and ubiquitously expressed in the mouse brain.[25] Proteins are localized in the human testis and thyroid.[26]
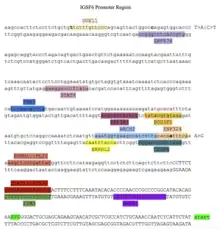
Promotor and Transcription Factors
The promotor region and transcription factors are shown in the promotor diagram. The transcription factors shown were highly conserved in animal orthologs of IGSF6.
Protein Level Regulation
The IGSF6 protein is predicted to be in the plasma membrane.[27][28] IGSF6 has a signal peptide from amino acids 17 to 32.[29] IGSF6 has post-translational modifications including phosphorylation sites and lysine acetylation sites. The phosphorylation sites at amino acid positions 3, 5, 91, 193, 198, 222, and 236, and these sites are important in enzymatic function.[30] The lysine acetylation sites are at amino acids 187, 195, 196, 213, and 224, and they are important in gene expression, protein–protein interactions, and protein processing and degradation.[31][32] IGSF6 has a SUMOylation site at amino acid 190.[33]
Homology and Evolution
Paralogs
The only paralog of IGSF6 is T cell receptor beta variable 28 (TCRBV28).[34] Birds are the most distant organism that TRBV28 is found in, so the gene duplication to create the paralog occurred about 320 million years ago.[35] TRBV28 is a quickly evolving gene, as it evolves similarly to fibrinogen alpha.
Orthologs
The orthologs of IGSF6 were found through NCBI protein and sorted by median date of divergence and sequence identity to the human protein.[36] The IGSF6 protein is found only in vertebrates with the H. sapiens IGSF6 protein being most distantly related to the fish IGSF6 protein. The human IGSF6 protein is most closely related to the IGSF6 protein of other mammals. Aves, reptiles, amphibians, and fish proteins have an average sequence similarity to the human protein of 52%, 50%, 50%, and 45% respectively.[37] IGSF6 is a fast-evolving gene because it evolves similarly to fibrinogen alpha.


Interacting Proteins
The most likely protein to interact with IGSF6 is methyltransferase-like protein 9 (METTL9) because IGSF6 is in an intron of METTL9. Most of the proteins that IGSF6 interacts with have immunological functions.[38]
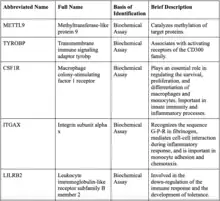
Clinical Significance
IGSF6 is predicted to be involved in immunological response. Its high expression in white blood cells and secondary lymphoid organs support this. IGSF6 has been associated with several diseases and conditions.
Inflammatory Bowel Disease
The human IGSF6 gene was localized to a locus associated with inflammatory bowel disease. IGSF6 has been researched as a possible indicator of inflammatory bowel disease (IBD) susceptibility.[39] However, there was no association with single nucleotide polymorphisms (SNPs) and IBD in patients with the disease.[40]
Esophageal Squamous Cell Carcinoma
The combined expression of IGSF6 and nine other genes was significantly related to the overall and disease-free survival in patients with esophageal squamous cell carcinoma.[41]
Multiple Sclerosis
IGSF6 was found to be upregulated in the myeloid cells function pathway in patients with multiple sclerosis, a chronic autoimmune demyelinating disease of the central nervous system.[42]
References
- GRCh38: Ensembl release 89: ENSG00000140749 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000035004 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Phyre Predicted Structure of IGSF6
- iCN3D .
- NCBI (National Library of Medicine) .
- NCBI (National Library of Medicine IGSF6 .
- NCBI (National Library of Medicine) Gene Entry on IGSF6 .
- King, K., Moody, A., Fisher, S. A., Mirza, M. M., Cuthbert, A. P., Hampe, J., Sutherland-Craggs, A., Sanderson, J., MacPherson, A. J., Forbes, A., Mansfield, J., Schreiber, S., Lewis, C. M., & Mathew, C. G. (2003). Genetic variation in the IGSF6 gene and lack of association with inflammatory bowel disease. European journal of immunogenetics : official journal of the British Society for Histocompatibility and Immunogenetics, 30(3), 187–190. https://doi.org/10.1046/j.1365-2370.2003.00387.x
- NCBI IGSF6 mRNA .
- UCSC Genome Browser IGSF6 .
- ExPASy pI/Mw for IGSF6 .
- ELM Prediction of IGSF6 Protein .
- I-Tasser Results for IGSF6 .
- I-Tasser Results for IGSF6 .
- Wei Zheng, Chengxin Zhang, Yang Li, Robin Pearce, Eric W. Bell, Yang Zhang. Folding non-homology proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Reports Methods, 1: 100014 (2021).
- Chengxin Zhang, Peter L. Freddolino, and Yang Zhang. COFACTOR: improved protein function prediction by combining structure, sequence and protein-protein interaction information. Nucleic Acids Research, 45: W291-299 (2017).
- Jianyi Yang, Yang Zhang. I-TASSER server: new development for protein structure and function predictions, Nucleic Acids Research, 43: W174-W181, 2015.
- SAPS analysis of IGSF6 .
- DiANNA predicted disulfide bonds for IGSF6 Archived 2022-07-24 at the Wayback Machine.
- NCBI Gene Entry on IGSF6 .
- NCBI Gene Entry on IGSF6 .
- NCBI GeoProfiles of IGSF6 .
- Allen Brain Atlas .
- ThermoFischer Scientific IGSF6 Antibody .
- PSORT II Prediction of IGSF6 .
- DeepLoc IGSF6 Prediction .
- NCBI (National Library of Medicine) .
- GPS Phosphorylation Sites .
- GPS-Pail Prediction of Acetylation .
- Zencheck, W. D., Xiao, H., & Weiss, L. M. (2012). Lysine post-translational modifications and the cytoskeleton. Essays in biochemistry, 52, 135–145. https://doi.org/10.1042/bse0520135
- GPS-SUMO Prediction of SUMOylation sites .
- NCBI Blast .
- TimeTree Divergence of Humans and Birds .
- NCBI Protein .
- Emboss Needle Alignment .
- String DB view of IGSF6 Protein interactions .
- Bates, E. E., Kissenpfennig, A., Péronne, C., Mattei, M. G., Fossiez, F., Malissen, B., & Lebecque, S. (2000). The mouse and human IGSF6 (DORA) genes map to the inflammatory bowel disease 1 locus and are embedded in an intron of a gene of unknown function. Immunogenetics, 52(1-2), 112–120. https://doi.org/10.1007/s002510000259
- King, K., Moody, A., Fisher, S. A., Mirza, M. M., Cuthbert, A. P., Hampe, J., Sutherland-Craggs, A., Sanderson, J., MacPherson, A. J., Forbes, A., Mansfield, J., Schreiber, S., Lewis, C. M., & Mathew, C. G. (2003). Genetic variation in the IGSF6 gene and lack of association with inflammatory bowel disease. European journal of immunogenetics : official journal of the British Society for Histocompatibility and Immunogenetics, 30(3), 187–190. https://doi.org/10.1046/j.1365-2370.2003.00387.x
- Guo, J., Liu, T., Shi, X., Mao, B., Zhang, J., Zhang, H., & Xian, L. (2020). Transcriptional profile of immune microenvironment and their prediction role for the prognosis of esophageal squamous cell carcinoma. Journal of Clinical Oncology, 38(4), 416–416. https://doi.org/10.1200/jco.2020.38.4_suppl.416
- Ivanova, M., Voronkova, A., Sukhorukov, V., & Zakharova, M. (2021). Different neuroinflammatory gene expression profiles in highly active and benign multiple sclerosis. Journal of Neuroimmunology, 358, 577650. https://doi.org/10.1016/j.jneuroim.2021.577650



