Dysidenin
Dysidenin is a toxin derived from the marine sponge Lamellodysidea herbacea and has been identified as lethal to certain fish species and other marine organisms. The toxic mechanism of dysidenin is linked to its ability to inhibit iodide transport in thyroid cells, which is a crucial process for thyroid hormone synthesis and subsequent metabolic regulation in organisms.[1] The inhibition of iodide transport could potentially lead to disrupted thyroid functions, causing a range of metabolic issues. This aspect of dysidenin not only sheds light on ecological interactions within marine environments but also suggests potential medical applications under controlled conditions.
 | |
| Names | |
|---|---|
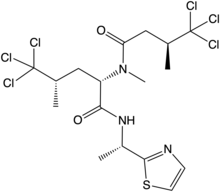
| Systematic IUPAC name
(2S,4S)-5,5,5-Trichloro-4-methyl-N-[(1S)-1-(1,3-thiazol-2-yl)ethyl]-2-[(3S)-4,4,4-trichloro-N,3-dimethylbutanamido]pentanamide | |
| Other names
Dysidenine;Pentanamide, 5,5,5-trichloro-4-methyl-2-(methyl(4,4,4-trichloro-3-methyl-1-oxobutyl)amino)-N-(1-(2-thiazolyl)ethyl)-;(2S,4S)-5,5,5-Trichloro-4-methyl-2-[methyl[(S)-4,4,4-trichloro-3-methyl-1-oxobutyl]amino]-N-[(S)-1-(2-thiazolyl)ethyl]pentanamide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C17H23Cl6N3O2S | |
| Molar mass | 546.166 |
| Appearance | Fine colorless needles |
| Melting point | 98° |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
There is a notable regional variation in the metabolites produced by Lamellodysidea herbacea, as evidenced by the isolation of dysidenin from samples obtained from the Great Barrier Reef, while it was absent in samples from the Caroline Islands.[2] This geographical variation in metabolite expression could be attributed to differences in environmental conditions, predator-prey interactions, or microbial symbiont communities within these regions. It highlights the adaptive metabolomic responses of marine sponges to their local ecological settings. This variance underscores the need for a deeper understanding of how spatial differences in marine ecosystems influence the chemical ecology of resident organisms.
The scientific exploration of dysidenin commenced in 1977 with its initial isolation.[3] Over the ensuing decades, the elucidation of dysidenin's structural and toxicological properties has contributed significantly to the broader field of marine natural products, which is a domain rich with potential pharmacological candidates and ecological indicators. The trajectory from its discovery to the present understanding exemplifies the gradual, yet rewarding, unraveling of marine-derived substances, mirroring the broader narrative of marine natural product research.
The discourse surrounding dysidenin serves as a reflection of the larger narrative on marine natural products, an arena filled with both potential and challenges. Dysidenin serves as a reminder of the delicate equilibrium within marine ecosystems and the inherent link between the health of marine habitats and the biodiscovery potential they hold. A nuanced understanding of dysidenin, both as an ecological factor and a potential biomedical resource, calls for a multidisciplinary approach anchored by rigorous analytical examination, and an appreciation for the complex, intertwined narratives of marine ecological and biochemical dynamics.
References
- Van Sande, J; Deneubourg, F; Beauwens, R; Braekman, JC; Daloze, D; Dumont, JE (April 1990). "Inhibition of iodide transport in thyroid cells by dysidenin, a marine toxin, and some of its analogs". Molecular Pharmacology. 37 (4): 583–9. PMID 2157965.
- Scheuer, Paul J., ed. (1978). Marine Natural Products V2 Chemical and Biological Perspectives. Oxford: Elsevier Science. p. 370. ISBN 9780323151863.
- Kazlauskas, R.; Lidgard, R.O.; Wells, R.J.; Vetter, W. (January 1977). "A novel hexachloro-metabolite from the sponge dysidea herbacea". Tetrahedron Letters. 18 (36): 3183–3186. doi:10.1016/S0040-4039(01)83192-0.