E-4 process
The E-4 process is a now outdated process for developing color reversal (transparency) photographic film, which was introduced in 1966.
- See also Ektachrome for full details of Kodak E-series processes.
Drawbacks
The process is infamous for two reasons:
First, it uses the highly toxic boron hydride-based reversal agent tertiary butyl-amine borane (TBAB).[lower-alpha 1][1]: 379, Table LXVI Early releases of the consumer-sized version of the chemistry provided the TBAB in the form of a tablet, possibly to avoid the possibility of inhalation.[2] This was later changed to loose powder, likely as a countermeasure against inadvertent ingestion of the substance.
Second, the prehardener agent contains formaldehyde and 2,5-dimethoxytetrahydrofuran,[1]: 377, Formula 269 which when mixed generates succinaldehyde, a noxious gas which has been likened to tear gas.[2] Process E-6 films are hardened during manufacture, eliminating the prehardener step altogether and allowing them to be processed at 100 °F (38 °C).
Steps
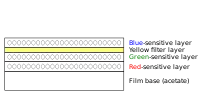
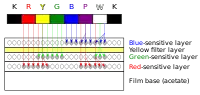
Ektachrome film has three separate light-sensitive layers; each layer is sensitive to a different group of wavelengths corresponding to red, green, and blue colors. When the film is exposed, each layer records a latent image based on its sensitivity. A yellow filter prevents blue light from exposing the green- and red-sensitive layers, which have some sensitivity to blue light.[3]
The E-4 process is faster than E-3; whereas E-3 required 15 steps and up to 70 minutes from start to finish,[2][4]: 30–31 E-4 was completed in approximately 50 minutes over 13 steps.[5] E-4 runs at 85 °F (29 °C),[5] about 10°F (6°C) higher than E-3. The temperature tolerance is ±1°F for prehardener, ±1⁄2°F for the first developer, and ±2–5°F for all other steps.[5] The ME-4 process was a motion picture variation of the E-4 process.
The major change for E-4 was the inclusion of a chemical reversal agent, which permits processing of the film without the manual re-exposure/fogging step required by the predecessor E-1 / E-2 / E-3 processes.[2][5]
Total darkness is required during the first four development steps; normal room light can be used for the remaining steps.[5]
| Step | Schematic | Time (min.) | Temp. | Description | ||
|---|---|---|---|---|---|---|
| 1 | Prehardener | 3 | 85 °F (29 °C) ±1°F | Tempers film for high-temperature processing | ||
| 2 | Neutralizer | 1 | 83–87 °F (28–31 °C) | |||
| 3 | First developer | 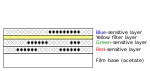 |
7 | 85 °F (29 °C) ±1⁄2°F | Conventional black-and-white developer used to transform silver halide crystals exposed in all three layers as a negative image. | |
| 4 | First stop bath | 2 | 83–87 °F (28–31 °C) | Solution should not be reused for second stop bath (step 7) | ||
| 5 | Wash | 4 | 80–90 °F (27–32 °C) | Running water | ||
| 6 | Color developer | 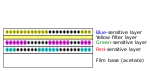 |
9 | 83–87 °F (28–31 °C) | ||
| 7 | Second stop bath | 3 | 83–87 °F (28–31 °C) | Solution should not be reused from first stop bath (step 4) | ||
| 8 | Wash | 3 | 80–90 °F (27–32 °C) | Running water | ||
| 9 | Bleach | 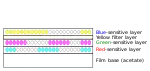 |
5 | 83–87 °F (28–31 °C) | Convert metallic silver to soluble particles | |
| 10 | Fixer |  |
6 | 83–87 °F (28–31 °C) | Dissolve silver particles, which can be recovered after processing | |
| 11 | Wash | 6 | 80–90 °F (27–32 °C) | Running water | ||
| 12 | Stabilizer | 1 | 83–87 °F (28–31 °C) | |||
| 13 | Dry | var. | <110 °F (43 °C) | |||
History

E-4 processed film is color stable for about 30 years.[6]
The process largely was phased out in 1976 with the introduction of the E-6 process, which is more environmentally friendly due to its lack of toxic chemicals. E-6 avoids the use of TBAB by adding a separate reversal bath containing the tin salt stannous chloride.
The E-4 process has been discontinued since 1996; after 1976 it was used solely for Kodak IE color infrared film,[7] due to a legal commitment by Kodak to provide process support for 30 years after introduction. Kodak discontinued E-4 processing in 1985, but independent photofinishers continued to support the process.[8] The E-4 chemicals were reverse-engineered and substitute formulae were published in the British Journal of Photography Annual in 1977.[1]: 374
Notes
- Not to be confused with tetra-n-butylammonium bromide, which also is abbreviated as TBAB.
References
- Jacobson, Kurt I.; Jacobson, Ralph Eric (1980). "Processing Colour Films". Developing: The Negative Technique (Eighteenth revised ed.). London: Focal Press. pp. 363–383. ISBN 0-240-44770-0. Retrieved 24 August 2023.
- Talbert, Michael. "Kodak Ektachrome Colour Transparency films". Photo Memorabilia. Retrieved 24 August 2023.
- "Process E-6 Using KODAK Chemicals, Process E-6 Publication Z-119 | Chapter 1: Processing solutions and their effects" (PDF). Kodak. Archived from the original (PDF) on August 25, 2005.
- Kodak Ektachrome Film, Publication No. E-13. Eastman Kodak Company. 1955.
- Wahl, Paul (April 1968). "Kodak's new E-4 kit: 50-Minute Cure for People Afraid to Develop Their Own Color Film". Popular Science. pp. 130–131.
- "Ektachrome: A Look Back". 25 January 2017.
- Ensanian, Armand (July 1988). "Inner Visions". Popular Mechanics. pp. 100–101. Retrieved 24 August 2023.
Color IR film has one drawback. It is not readily processed because it requires the old E-4 chemistry.
- Rothschild, Norman (December 1985). "Pop Photo Snapshots: Bad and good news from Kodak". Popular Photography. pp. 28–32, 114. Retrieved 24 August 2023.
Eastman Kodak no longer offers processing for E-4 films such as Ektachrome Infrared and Kodak Microphotography color-slide films. However, there are more than a dozen independent labs in the U.S. that offer this service.
External links
- Kodak specifications for hand mixing of chemistry
- "More than you want to know about E-4". 19 May 1995.
Processing of older Ektachrome films (including Process E-4)
- Film Rescue USA and Canada
- Rocky Mountain USA