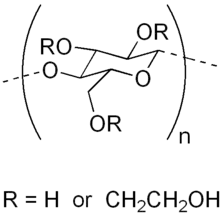
Hydroxyethyl cellulose
Hydroxyethyl cellulose is a gelling and thickening agent derived from cellulose. It is widely used in cosmetics, cleaning solutions, and other household products.[1] Hydroxyethyl cellulose and methyl cellulose are frequently used with hydrophobic drugs in capsule formulations, to improve the drugs' dissolution in the gastrointestinal fluids. This process is known as hydrophilization.[2]
 | |
 | |
| Names | |
|---|---|
| Other names
Cellulose, hydroxyethyl ether; Hydroxyethylcellulose; 2-Hydroxyethyl cellulose; Hyetellose; Natrosol; Cellosize | |
| Identifiers | |
| ChEBI | |
| ChemSpider |
|
| ECHA InfoCard | 100.116.562 |
| E number | E1525 (additional chemicals) |
| UNII | |
CompTox Dashboard (EPA) |
|
| Properties | |
| variable | |
| Molar mass | variable |
| Melting point | 140 °C (284 °F; 413 K) |
| Hazards | |
| Safety data sheet (SDS) | MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Hydroxyethyl cellulose is also used extensively in the oil and gas industry as a drilling mud additive under the name HEC as well in industrial applications, paint and coatings, ceramics, adhesives, emulsion polymerization, inks, construction, welding rods, pencils and joint fillers.
Hydroxyethyl cellulose can be one of the main ingredients in water-based personal lubricants. It is also a key ingredient in the formation of large bubbles as it possesses the ability to dissolve in water but also provide structural strength to the soap bubble. Among other similar chemicals, it is often used as slime (and gunge, in the UK).
Hydroxyethyl cellulose (HEC) is a commonly used thickener in paint & coating formulations. HEC thickeners are used in paint & coating formulations to increase the viscosity of the paint and to improve its flow and leveling properties.[3]
References
- Hydroxyethyl cellulose in the Consumer Product Information Database
- "Natrosol hydroxyethylcellulose". ashland.com. Retrieved 12 May 2018.
- "KimaCell Hydroxyethyl Cellulose HEC". kimachemical.com. Retrieved 1 March 2023.