Hepatitis C virus envelope glycoprotein E1
E1 is one of two subunits of the envelope glycoprotein[1] found in the hepatitis C virus.[2][3] The other subunit is E2. This protein is a type 1 transmembrane protein with a highly glycosylated N-terminal ectodomain and a C-terminal hydrophobic anchor. After being synthesized the E1 glycoproteins associates with the E2 glycoprotein as a noncovalent heterodimer.[4]
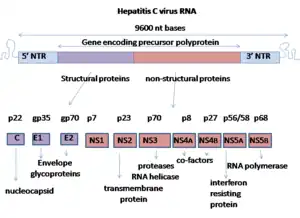
Structure
The E1 glycoprotein residues 192-383 in the genotype 1a H77 strain. After translation the E1 C-terminal transmembrane domains (TMDs) forms a hairpin of antiparallel a-helices. E1 is then cleaved by signal peptide peptidase at the endoplasmic reticulum and E1 is then made into a single long straight a-helix. What is known of the structure is from a crystal structure made in 2014.[5] This crystal structure shows that it has two a-helixes and 3 B-sheets for both monomers; two disulfide bridges stabilize these two monomers. This means that E1 is more compact then its E2 counterpart. It has been shown that E1 can fold with a small amount of E2 protein present. In addition to this it was shown that E1 oxidation preceded E2 maturation. This means that E1 has a chaperone-like role for E2.[6] Despite these finds there are many things still unknown about the structure of E1. The E1 protein is anchored to the membrane. Most of the time E1 remains in its unfolded conformation.
Function
The E1 protein helps the virus attach to the membrane of the targeted cell. In other envelope virus the E1 protein has a similar role in helping the virus get into the cell. As a heterodimer with E2 it has been discovered that it is essential for HCV entry.[7] When the heterodimer is formed the hepatitis C virus is then able to bind to the receptor of the cell. As a heterodimer the E1 protein alone with the E2 protein worked together to enter the cell. Also cleavage at the core-E1 junction is a prerequisite for SPP-catalyzed cleavage. This helps the virus relocate to the surface of lipid droplets. Once the virus gets to the surface of the lipid droplets it recruits the virus no-structural proteins and replication complex.[8] The SP-catalyzed cleavage at the core-E1 junction is required for the formation of infectious particles and for the release of any HCV particles. Also E1 has no function with budding at the ER membrane. It also had no effect on the intracellular formation of capsid-containing particles. Instead when E1 was not allowed to form this tended to facilitate the budding process.
Possible Vaccine
It has been shown that by blocking E1 we can prevent the formation of the envelope protein. There have been a number of studies trying to find the structure of E1. The hope for these vaccines is that they will be able to block the entry of Hepatitis C if they can block the formation of E1. If the virus cannot make the envelope protein then it will be unable to get into the host cells.[9] The types of vaccines that would be used are synthetic peptide vaccines.[10]
References
- Haddad, J.G.; Rouille, Y.; et al. (2017). "Identification of Novel Functions for Hepatitis C virus Envelope Glycoprotein E1 in Virus Entry and Assembly". Journal of Virology. 91 (11): e00048-17. doi:10.1128/JVI.00048-17. PMC 5375667. PMID 28179528.
- Garcia JE, Puentes A, Súarez J, et al. (February 2002). "Hepatitis C virus (HCV) E1 and E2 protein regions that specifically bind to HepG2 cells". J. Hepatol. 36 (2): 254–62. doi:10.1016/S0168-8278(01)00262-8. PMID 11830338.
- Bartosch B, Dubuisson J, Cosset FL (March 2003). "Infectious Hepatitis C Virus Pseudo-particles Containing Functional E1–E2 Envelope Protein Complexes". J. Exp. Med. 197 (5): 633–42. doi:10.1084/jem.20021756. PMC 2193821. PMID 12615904.
- Lavie, M.; Goffard, A.; Dubuisson, J. In Chapter 4 HCV Glycoproteins: Assembly of a Functional E1-E2 heterodimer; Norfolk: UK, 2006; .
- Freedman, H.; Logan, M. R.; Law, J. L.; Houghton, M. (2016). "Structure and Function of the Hepatitis C Virus Envelope Glycoproteins E1 and E2: Antiviral and Vaccine Targets". ACS Infectious Diseases. 2 (11): 749–762. doi:10.1021/acsinfecdis.6b00110. PMID 27933781.
- Abdelwahab, K. S.; Ahmed Said, Z. N. (2016). "Status of hepatitis C virus vaccination: Recent update". World Journal of Gastroenterology. 22 (2): 862–73. doi:10.3748/wjg.v22.i2.862. PMC 4716084. PMID 26811632.
- Lavie, M.; Goffard, A.; Dubuisson, J. In Chapter 4 HCV Glycoproteins: Assembly of a Functional E1-E2 heterodimer; Norfolk: UK, 2006; .
- Pène, V.; Lemasson, M.; Harper, F.; Pierron, G.; Rosenberg, A. R. (2017). "Role of cleavage at the core-E1 junction of hepatitis C virus polyprotein in viral morphogenesis". PLOS ONE. 12 (4): e0175810. Bibcode:2017PLoSO..1275810P. doi:10.1371/journal.pone.0175810. PMC 5402940. PMID 28437468.
- Freedman, H.; Logan, M. R.; Law, J. L.; Houghton, M. (2016). "Structure and Function of the Hepatitis C Virus Envelope Glycoproteins E1 and E2: Antiviral and Vaccine Targets". ACS Infectious Diseases. 2 (11): 749–762. doi:10.1021/acsinfecdis.6b00110. PMID 27933781.
- Abdelwahab, K. S.; Ahmed Said, Z. N. (2016). "Status of hepatitis C virus vaccination: Recent update". World Journal of Gastroenterology. 22 (2): 862–73. doi:10.3748/wjg.v22.i2.862. PMC 4716084. PMID 26811632.