Ascorbyl palmitate
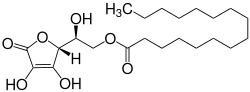
Ascorbyl palmitate is an ester formed from ascorbic acid and palmitic acid creating a fat-soluble form of vitamin C. In addition to its use as a source of vitamin C, it is also used as an antioxidant food additive (E number E304). It is approved for use as a food additive in the EU,[1] the U.S.,[2] Canada,[3] Australia, and New Zealand.[4]
 | |
| Names | |
|---|---|
| IUPAC name
L-threo-Hex-2-enono-1,4-lactone 6-hexadecanoate | |
| Systematic IUPAC name
(2S)-2-[(2R)-3,4-Dihydroxy-5-oxo-2,5-dihydrofuran-2-yl]-2-hydroxyethyl hexadecanoate | |
| Other names
Ascorbyl palmitate L-Ascorbic acid 6-hexadecanoate 6-O-Palmitoylascorbic acid | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | E304 |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.824 |
| E number | E304 (antioxidants, ...) |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C22H38O7 | |
| Molar mass | 414.539 g·mol−1 |
| Appearance | White to yellowish colored powder |
| Melting point | 116 to 117 °C (241 to 243 °F; 389 to 390 K) |
| Very slightly soluble in water; freely soluble in ethanol | |
| Hazards | |
| Flash point | 178.1 °C (352.6 °F; 451.2 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Ascorbyl palmitate is also marketed as "vitamin C ester". It is synthesized by acylation of vitamin C using different acyl donors.[5]
See also
References
- UK Food Standards Agency: "Current EU approved additives and their E Numbers". Retrieved 2011-10-27.
- US Food and Drug Administration: "Listing of Food Additives Status Part I". Food and Drug Administration. Archived from the original on 2012-01-17. Retrieved 2011-10-27.
- Health Canada: "Chemical Substance - Ascorbyl palmitate". 26 July 2004. Retrieved 2016-08-13.
- Australia New Zealand Food Standards Code"Standard 1.2.4 - Labelling of ingredients". Retrieved 2011-10-27.
- Karmee SK (January 2009). "Biocatalytic synthesis of ascorbyl esters and their biotechnological applications". Applied Microbiology and Biotechnology. 81 (6 Suppl): 1013–1022. doi:10.1007/s00253-008-1781-y. PMID 19030854. S2CID 35465409.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.