Erythorbic acid
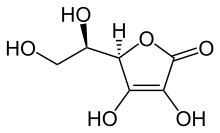
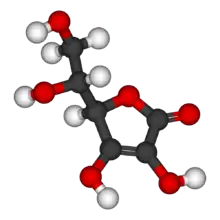
Erythorbic acid (isoascorbic acid, D-araboascorbic acid) is a stereoisomer of ascorbic acid (vitamin C).[1] It is synthesized by a reaction between methyl 2-keto-D-gluconate and sodium methoxide. It can also be synthesized from sucrose or by strains of Penicillium that have been selected for this feature.[2] It is denoted by E number E315, and is widely used as an antioxidant in processed foods.[3]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
D-erythro-Hex-2-enono-1,4-lactone | |
| Systematic IUPAC name
(5R)-5-[(1R)-1,2-Dihydroxyethyl]-3,4-dihydroxyfuran-2(5H)-one | |
| Other names
D-Araboascorbic acid, Erythorbate, Isoascorbic acid. | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.753 |
| E number | E315 (antioxidants, ...) |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H8O6 | |
| Molar mass | 176.124 g·mol−1 |
| Density | 0.704 g/cm3 |
| Melting point | 164 to 172 °C (327 to 342 °F; 437 to 445 K) (decomposes) |
| Acidity (pKa) | 2.1 |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other cations |
Calcium erythorbate, sodium erythorbate, potassium erythorbate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Clinical trials have been conducted to investigate aspects of the nutritional value of erythorbic acid. One such trial investigated the effects of erythorbic acid on vitamin C metabolism in young women; no effect on vitamin C uptake or clearance from the body was found.[4] A later study found that erythorbic acid is a potent enhancer of nonheme-iron absorption.[5]
Since the U.S. Food and Drug Administration banned the use of sulfites as a preservative in foods intended to be eaten fresh (such as salad bar ingredients), the use of erythorbic acid as a food preservative has increased.
It is also used as a preservative in cured meats and frozen vegetables.[6]
It was first synthesized in 1933 by the German chemists Kurt Maurer and Bruno Schiedt.[7][8]
References
- Erythorbic acid and its sodium salt Dr R. Walker, Professor of Food Science, Department of Biochemistry, University of Surrey, England.
- "Erythorbic acid".
- Current EU approved additives and their E Numbers, Food Standards Agency
- Sauberlich, HE; Tamura T; Craig CB; Freeberg LE; Liu T (September 1996). "Effects of erythorbic acid on vitamin C metabolism in young women". American Journal of Clinical Nutrition. 64 (3): 336–46. doi:10.1093/ajcn/64.3.336. PMID 8780343.
- Fidler, MC; Davidsson L; Zeder C; Hurrell RF (January 2004). "Erythorbic acid is a potent enhancer of nonheme-iron absorption". American Journal of Clinical Nutrition. 79 (1): 99–102. doi:10.1093/ajcn/79.1.99. PMID 14684404.
- Hui YH (2006). Handbook of Food Science, Technology and Engineering. CRC Press. pp. 83–32. ISBN 0-8493-9848-7.
- See:
- Maurer, Kurt; Schiedt, Bruno (August 2, 1933). ""Die Darstellung einer Säure C6H8O6 aus Glucose, die in ihrer Reduktionskraft der Ascorbinsäure gleicht (Vorläuf. Mitteil.)" (The preparation of an acid C6H8O6 from glucose, which equals ascorbic acid in its reducing power (preliminary report))". Berichte der Deutschen Chemischen Gesellschaft. 66 (8): 1054–1057. doi:10.1002/cber.19330660807.
- Maurer, Kurt; Schiedt, Bruno (July 4, 1934). ""Zur Darstellung des Iso-Vitamins C (d-Arabo-ascorbinsäure) (II. Mitteil.)" (On the preparation of iso-vitamin C (d-arabo-ascorbic acid) (2nd report))". Berichte der Deutschen Chemischen Gesellschaft. 67 (7): 1239–1241. doi:10.1002/cber.19340670724.
- See also:
- Ohle, Heinz; Erlbach, Heinz; Carls, Herbert (February 7, 1934). ""d-Gluco-saccharosonsäure, ein Isomeres der Ascorbinsäure, I. Mitteil.: Darstellung und Eigenschaften" (d-Gluco-saccharosonic acid, an isomer of ascorbic acid, 1st report: preparation and properties)". Berichte der Deutschen Chemischen Gesellschaft. 67 (2): 324–332. doi:10.1002/cber.19340670235.
- Baird, D. K.; Haworth, W. N.; Herbert, R. W.; Hirst, E. L.; Smith, F.; Stacey, M. (1934). "Ascorbic acid and synthetic analogues". Journal of the Chemical Society: 63–67. doi:10.1039/JR9340000062.
- Reichstein, T.; Grüssner, A.; Oppenauer, R. (1934). ""Synthese der Ascorbinsäure und verwandter Verbindungen nach der Oson-Blausäure-Methode"(Synthesis of ascorbic acid and related compounds via the ozone-hydrogen cyanide method)". Helvetica Chimica Acta. 17: 510–520. doi:10.1002/hlca.19340170157.
