Carboxymethyl cellulose
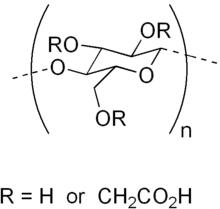
Carboxymethyl cellulose (CMC) or cellulose gum[1] is a cellulose derivative with carboxymethyl groups (-CH2-COOH) bound to some of the hydroxyl groups of the glucopyranose monomers that make up the cellulose backbone. It is often used as its sodium salt, sodium carboxymethyl cellulose. It used to be marketed under the name Tylose, a registered trademark of SE Tylose.[2]
 | |
 | |
| Names | |
|---|---|
| Other names
Carboxymethylcellulose; carmellose; E466 | |
| Identifiers | |
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.120.377 |
| E number | E466 (thickeners, ...) |
| UNII | |
CompTox Dashboard (EPA) |
|
| Properties | |
| variable | |
| Molar mass | variable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation
Carboxymethyl cellulose is synthesized by the alkali-catalyzed reaction of cellulose with chloroacetic acid.[3] The polar (organic acid) carboxyl groups render the cellulose soluble and chemically reactive.[4] Fabrics made of cellulose—e.g. cotton or viscose rayon—may also be converted into CMC.
Following the initial reaction, the resultant mixture produces approximately 60% CMC and 40% salts (sodium chloride and sodium glycolate); this product is the so-called technical CMC, which is used in detergents. An additional purification process is used to remove salts to produce pure CMC, which is used for alimentary and pharmaceutical applications. An intermediate "semi-purified" grade is also produced, typically used in paper applications such as the restoration of archival documents.
Structure and properties
The functional properties of CMC depend on the degree of substitution of the cellulose structure [i.e., how many of the hydroxyl groups have been converted to carboxymethylene(oxy) groups in the substitution reaction], as well as the chain length of the cellulose backbone structure and the degree of clustering of the carboxymethyl substituents.
Uses
Introduction
Carboxymethyl cellulose (CMC) is used in a variety of applications ranging from food production to medical treatments.[5] It is commonly used as a viscosity modifier or thickener, and to stabilize emulsions in various products, both food and non-food. It is used primarily because it has high viscosity, is nontoxic, and is generally considered to be hypoallergenic, as the major source fiber is either softwood pulp or cotton linter. Non-food products include products such as toothpaste, laxatives, diet pills, water-based paints, detergents, textile sizing, reusable heat packs, various paper products, filtration materials, synthetic membranes, wound healing applications, and also in leather crafting to help burnish edges.[6][7][8]
Food science
CMC is used in food under the E number E466 or E469 (when it is enzymatically hydrolyzed), as a viscosity modifier or thickener, and to stabilize emulsions in various products, including ice cream.[7][6] CMC is also used extensively in gluten-free and reduced-fat food products.[9]
CMC is used to achieve tartrate or cold stability in wine, an innovation that may save megawatts of electricity used to chill wine in warm climates. It is more stable than metatartaric acid and is very effective in inhibiting tartrate precipitation. It is reported that KHT crystals, in presence of CMC, grow slower and change their morphology.[10] Their shape becomes flatter because they lose 2 of the 7 faces, changing their dimensions. CMC molecules, negatively charged at wine pH, interact with the electropositive surface of the crystals, where potassium ions are accumulated. The slower growth of the crystals and the modification of their shape are caused by the competition between CMC molecules and bitartrate ions for binding to the KHT crystals.[11]
Specific culinary uses
CMC powder is widely used in the ice cream industry, to make ice creams without churning or extremely low temperatures, thereby eliminating the need for conventional churners or salt ice mixes.[12] CMC is used in baking breads and cakes. The use of CMC gives the loaf an improved quality at a reduced cost, by reducing the need of fat. CMC is also used as an emulsifier in biscuits. By dispersing fat uniformly in the dough, it improves the release of the dough from the moulds and cutters, achieving well-shaped biscuits without any distorted edges. It can also help to reduce the amount of egg yolk or fat used in making the biscuits. Use of CMC in candy preparation ensures smooth dispersion in flavor oils, and improves texture and quality. CMC is used in chewing gums, margarines and peanut butter as an emulsifier.[13]
Medical applications
CMC is also used in numerous medical applications.[14][15][16][17]
Some examples include:
- Device for epistaxis (nose bleeding). A poly-vinyl chloride (PVC) balloon is covered by CMC knitted fabric reinforced by nylon. The device is soaked in water to form a gel, which is inserted into the nose of the balloon and inflated. The combination of the inflated balloon and the therapeutic effect of the CMC stops the bleeding.
- Fabric used as a dressing following ear nose and throat surgical procedures.
- Water is added to form a gel, and this gel is inserted into the sinus cavity following surgery.
In ophthalmology, CMC is used as a lubricating agent in artificial tears solutions for the treatment of dry eyes.[18]
In veterinary medicine, CMC is used in abdominal surgeries in large animals, particularly horses, to prevent the formation of bowel adhesions.
Research applications
Insoluble CMC (water-insoluble) can be used in the purification of proteins, particularly in the form of charged filtration membranes or as granules in cation-exchange resins for ion-exchange chromatography.[19] Its low solubility is a result of a lower DS value (the number of carboxymethyl groups per anhydroglucose unit in the cellulose chain) compared to soluble CMC.[20] Insoluble CMC offers physical properties similar to insoluble cellulose, while the negatively charged carboxylate groups allow it to bind to positively charged proteins.[21] Insoluble CMC can also be chemically cross-linked to enhance the mechanical strength of the material.[22]
Moreover, CMC has been used extensively to characterize enzyme activity from endoglucanases (part of the cellulase complex); it is a highly specific substrate for endo-acting cellulases, as its structure has been engineered to decrystallize cellulose and create amorphous sites that are ideal for endoglucanase action. CMC is desirable because the catalysis product (glucose) is easily measured using a reducing sugar assay, such as 3,5-dinitrosalicylic acid. Using CMC in enzyme assays is especially important in screening for cellulase enzymes that are needed for more efficient cellulosic ethanol conversion. CMC was misused in early work with cellulase enzymes, as many had associated whole cellulase activity with CMC hydrolysis. As the mechanism of cellulose depolymerization became better understood, it became clear that exo-cellulases are dominant in the degradation of crystalline (e.g. Avicel) and not soluble (e.g. CMC) cellulose.
Other uses
In laundry detergents, it is used as a soil suspension polymer designed to deposit onto cotton and other cellulosic fabrics, creating a negatively charged barrier to soils in the wash solution. CMC is also used as a thickening agent, for example, in the oil-drilling industry as an ingredient of drilling mud, where it acts as a viscosity modifier and water retention agent.
CMC is sometimes used as an electrode binder in advanced battery applications (i.e. lithium ion batteries), especially with graphite anodes.[23] CMC's water solubility allows for less toxic and costly processing than with non-water-soluble binders, like the traditional polyvinylidene fluoride (PVDF), which requires toxic n-methylpyrrolidone (NMP) for processing. CMC is often used in conjunction with styrene-butadiene rubber (SBR) for electrodes requiring extra flexibility, e.g. for use with silicon-containing anodes.[24]
CMC is also used in ice packs to form a eutectic mixture resulting in a lower freezing point, and therefore more cooling capacity than ice.[25]
Aqueous solutions of CMC have also been used to disperse carbon nanotubes, where the long CMC molecules are thought to wrap around the nanotubes, allowing them to be dispersed in water.
In conservation-restoration, it is used as an adhesive or fixative (commercial name Walocel, Klucel).
Adverse reactions
Effects on inflammation, microbiota-related metabolic syndrome, and colitis are a subject of research.[26] Carboxymethyl cellulose is suggested as a possible cause of inflammation of the gut, through alteration of the human gastrointestinal microbiota, and has been suggested as a triggering factor in inflammatory bowel diseases such as ulcerative colitis and Crohn's disease.[27]
While thought to be uncommon, case reports of severe reactions to carboxymethyl cellulose exist.[28] Skin testing is believed to be a useful diagnostic tool for this purpose.[29] Carboxymethyl cellulose was the active ingredient in an eye drop brand Ezricare Artificial Tears which was recalled due to potential bacterial contamination.[30]
References
- Codex Alimentarius Commission (2016). "Sodium carboxymethyl cellulose (Cellulose gum)". GFSA Online. FAO.
- "Products – SE Tylose". www.setylose.com. Retrieved 2022-11-17.
- Hollabaugh, C. B.; Burt, Leland H.; Walsh, Anna Peterson (October 1945). "Carboxymethylcellulose. Uses and Applications". Industrial & Engineering Chemistry. 37 (10): 943–947. doi:10.1021/ie50430a015.
- "CMC Sodium Carboxymethylcellulose" (PDF). colonygums.com. Retrieved 19 May 2023.
- Rahman, Md Saifur; Hasan, Md Saif; Nitai, Ashis Sutradhar; Nam, Sunghyun; Karmakar, Aneek Krishna; Ahsan, Md Shameem; Shiddiky, Muhammad J. A.; Ahmed, Mohammad Boshir (2021). "Recent Developments of Carboxymethyl Cellulose". Polymers. 13 (8): 1345. doi:10.3390/polym13081345. ISSN 2073-4360. PMC 8074295. PMID 33924089.
- "CP Kelco Cellulose Gum / Carboxymethyl Cellulose". Archived from the original on 2013-08-24. Retrieved 2013-07-17.
- "Sodium Carboxymethylcellulose – The Ideal Hydrocolloid for Bakery & Dough Products" (PDF). Archived from the original (PDF) on 2015-06-26.
- Tudoroiu, Elena-Emilia; Dinu-Pîrvu, Cristina-Elena; Albu Kaya, Mădălina Georgiana; Popa, Lăcrămioara; Anuța, Valentina; Prisada, Răzvan Mihai; Ghica, Mihaela Violeta (2021). "An Overview of Cellulose Derivatives-Based Dressings for Wound-Healing Management". Pharmaceuticals. 14 (12): 1215. doi:10.3390/ph14121215. ISSN 1424-8247. PMC 8706040. PMID 34959615.
- Stanford, John (January 2012). "Food Processing Technologies for Reduction of Fat in Products" (PDF). Food & Health Innovation Service. Scotland Food & Drink. Archived from the original (PDF) on 2014-10-23.
- Gerbaud, Vincent (18 October 1996). Determination de l'etat de sursaturation et effet des polysaccharides sur la cristallisation du bitartrate de potassium dans les vins [Determination of the state of supersaturation and effect of polysaccharides on the crystallization of potassium bitartrate in wines] (PDF) (Ph.D.) (in French). Institut National Polytechnique de Talouse. Docket 961NP1030G. Retrieved 2017-05-07.
- Cracherau et al. 2001.
- Bahramparvar, Maryam; Mazaheri Tehrani, Mostafa (October 2011). "Application and Functions of Stabilizers in Ice Cream". Food Reviews International. 27 (4): 389–407. doi:10.1080/87559129.2011.563399. S2CID 43187328.
- "C.m.c. Glossary – Recipes with C.m.c. - Tarladalal.com". Retrieved 9 November 2016.
- Rahman, Md Saifur; Hasan, Md Saif; Nitai, Ashis Sutradhar; Nam, Sunghyun; Karmakar, Aneek Krishna; Ahsan, Md Shameem; Shiddiky, Muhammad J. A.; Ahmed, Mohammad Boshir (2021). "Recent Developments of Carboxymethyl Cellulose". Polymers. 13 (8): 1345. doi:10.3390/polym13081345. ISSN 2073-4360. PMC 8074295. PMID 33924089.
- Tudoroiu, Elena-Emilia; Dinu-Pîrvu, Cristina-Elena; Albu Kaya, Mădălina Georgiana; Popa, Lăcrămioara; Anuța, Valentina; Prisada, Răzvan Mihai; Ghica, Mihaela Violeta (2021). "An Overview of Cellulose Derivatives-Based Dressings for Wound-Healing Management". Pharmaceuticals. 14 (12): 1215. doi:10.3390/ph14121215. ISSN 1424-8247. PMC 8706040. PMID 34959615.
- Zennifer, Allen; Senthilvelan, Praseetha; Sethuraman, Swaminathan; Sundaramurthi, Dhakshinamoorthy (2021-03-15). "Key advances of carboxymethyl cellulose in tissue engineering & 3D bioprinting applications". Carbohydrate Polymers. 256: 117561. doi:10.1016/j.carbpol.2020.117561. ISSN 0144-8617. PMID 33483063. S2CID 231689461.
- Ciolacu, Diana Elena; Nicu, Raluca; Ciolacu, Florin (2020). "Cellulose-Based Hydrogels as Sustained Drug-Delivery Systems". Materials. 13 (22): 5270. doi:10.3390/ma13225270. ISSN 1996-1944. PMC 7700533. PMID 33233413.
- "Carboxymethylcellulose: Indications, Side Effects, Warnings". Drugs.com. Retrieved 2023-08-08.
- "Whatman Filters & Sample Collection". Archived from the original on 2 May 2013. Retrieved 9 November 2016.
- Wang, Mengying; Jia, Xiangxiang; Liu, Wanshuang; Lin, Xiaobo (2021-03-01). "Water insoluble and flexible transparent film based on carboxymethyl cellulose". Carbohydrate Polymers. 255: 117353. doi:10.1016/j.carbpol.2020.117353. ISSN 0144-8617. PMID 33436193. S2CID 228813982.
- Lopez, Carlos G.; Colby, Ralph H.; Cabral, João T. (2018-04-24). "Electrostatic and Hydrophobic Interactions in NaCMC Aqueous Solutions: Effect of Degree of Substitution". Macromolecules. 51 (8): 3165–3175. doi:10.1021/acs.macromol.8b00178. ISSN 0024-9297.
- Nakayama, Ryo-ichi; Yano, Tomoya; Namiki, Norikazu; Imai, Masanao (2019-11-01). "Highly Size-Selective Water-Insoluble Cross-Linked Carboxymethyl Cellulose Membranes". Journal of Polymers and the Environment. 27 (11): 2439–2444. doi:10.1007/s10924-019-01532-w. ISSN 1572-8919. S2CID 199474275.
- Park, Jeong Hoon; Kim, Sun Hyung; Ahn, Kyung Hyun (2023-05-05). "Role of carboxymethyl cellulose binder and its effect on the preparation process of anode slurries for Li-ion batteries". Colloids and Surfaces A: Physicochemical and Engineering Aspects. 664: 131130. doi:10.1016/j.colsurfa.2023.131130. ISSN 0927-7757. S2CID 256917952.
- Applications of sodium carboxymethyl cellulose As a Binder In Batteries
- "Use in ice packs". Archived from the original on July 8, 2011.
- Healy, Melissa (2015-02-25). "Is common food additive to blame for rising rates of bowel disease?". Los Angeles Times. Archived from the original on 2017-07-12. Retrieved 2017-07-12.
- Martino, John Vincent; Van Limbergen, Johan; Cahill, Leah E. (1 May 2017). "The Role of Carrageenan and Carboxymethylcellulose in the Development of Intestinal Inflammation". Frontiers in Pediatrics. 5: 96. doi:10.3389/fped.2017.00096. PMC 5410598. PMID 28507982.
- Chassaing, Benoit; Compher, Charlene; Bonhomme, Brittaney; Liu, Qing; Tian, Yuan; Walters, William; Nessel, Lisa; Delaroque, Clara; Hao, Fuhua; Gershuni, Victoria; Chau, Lillian; Ni, Josephine; Bewtra, Meenakshi; Albenberg, Lindsey; Bretin, Alexis; McKeever, Liam; Ley, Ruth E.; Patterson, Andrew D.; Wu, Gary D.; Gewirtz, Andrew T.; Lewis, James D. (11 November 2021). "Randomized Controlled-Feeding Study of Dietary Emulsifier Carboxymethylcellulose Reveals Detrimental Impacts on the Gut Microbiota and Metabolome". Gastroenterology. 162 (3): 743–756. doi:10.1053/j.gastro.2021.11.006. PMC 9639366. PMID 34774538.
- Lieberman, Phil. "Anaphylaxis to carboxymethylcellulose". American Academy of Allergy, Asthma, and Immunology. Archived from the original on 2017-07-12. Retrieved 2017-07-12.
- "Drug regulatory body takes eye drop samples from pharma firm linked to US deaths". 4 February 2023.