Ectoine
 | |
| Names | |
|---|---|
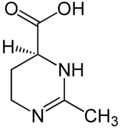
| Preferred IUPAC name
(4S)-2-Methyl-3,4,5,6-tetrahydropyrimidine-4-carboxylic acid | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H10N2O2 | |
| Molar mass | 142.158 g·mol−1 |
| Appearance | White powder |
| Density | 1.568 g/cm3 |
| Soluble in water | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Ectoine (1,4,5,6-tetrahydro-2-methyl-4-pyrimidinecarboxylic acid) is a natural compound found in several species of bacteria. It is a compatible solute which serves as a protective substance by acting as an osmolyte and thus helps organisms survive extreme osmotic stress. Ectoine is found in high concentrations in halophilic microorganisms and confers resistance towards salt and temperature stress. Ectoine was first identified in the microorganism Ectothiorhodospira halochloris,[3][4] but has since been found in a wide range of Gram-negative and Gram-positive bacteria. Other species of bacteria in which ectoine was found include:
Biosynthesis
Ectoine is synthesized in three successive enzymatic reactions starting from aspartic β-semialdehyde. The genes involved in the biosynthesis are called ectA, ectB and ectC, and they encode the enzymes L-2,4-diaminobutyric acid acetyltransferase, L-2,4-diaminobutyric acid transaminase and L-ectoine synthase, respectively.[6][5]
Use in cosmetics
Ectoine is used as an active ingredient in skin care and sun protection products.[11] It stabilizes proteins and other cellular structures and protects the skin from stresses like UV irradiation and dryness.[5]
Medical use
Due to its protein stabilizing properties, ectoine has been evaluated as a topical treatment for hay fever. Effectiveness of a nasal spray containing ectoine is comparable to cromoglycate and is reported to be well tolerated by the patients.[12] It is available over-the-counter in European Union.
References
- Ectoine at Sigma-Aldrich
- Ectoine SDS
- Peters, P; Miwatani, T; Honda, T (1990). "The biosynthesis of ectoine". FEMS Microbiology Letters. 71 (2): 157–61. doi:10.1016/0378-1097(90)90049-V. PMID 1601286.
- Bernard, T.; Jebbar, M.; Rassouli, Y.; Himdi-Kabbab, S.; Hamelin, J.; Blanco, C. (1993). "Ectoine accumulation and osmotic regulation in Brevibacterium linens" (PDF). Journal of General Microbiology. 139: 129–136. doi:10.1099/00221287-139-1-129.
- Stöveken, N; Pittelkow, M; Sinner, T; Jensen, R. A.; Heider, J; Bremer, E (2011). "A specialized aspartokinase enhances the biosynthesis of the osmoprotectants ectoine and hydroxyectoine in Pseudomonas stutzeri A1501". Journal of Bacteriology. 193 (17): 4456–68. doi:10.1128/JB.00345-11. PMC 3165526. PMID 21725014.
- Louis, P; Galinski, E. A. (1997). "Characterization of genes for the biosynthesis of the compatible solute ectoine from Marinococcus halophilus and osmoregulated expression in Escherichia coli". Microbiology. 143 ( Pt 4) (4): 1141–9. doi:10.1099/00221287-143-4-1141. PMID 9141677.
- Augenstein, Seth (6 September 2016). "'Extremophile Bacteria' Will Eat Away Wreck of the Titanic". laboratoryequipment.com.
- Zaccai, Giuseppe; Bagyan, Irina; Combet, Jérôme; Cuello, Gabriel J.; Demé, Bruno; Fichou, Yann; Gallat, François-Xavier; Galvan Josa, Victor M.; von Gronau, Susanne; Haertlein, Michael; Martel, Anne; Moulin, Martine; Neumann, Markus; Weik, Martin; Oesterhelt, Dieter (16 August 2016). "Neutrons describe ectoine effects on water H-bonding and hydration around a soluble protein and a cell membrane". Scientific Reports. 6: 31434. Bibcode:2016NatSR...631434Z. doi:10.1038/srep31434. PMC 4985633. PMID 27527336.
- "HAMAP: Halorhodospira halophila (strain DSM 244 / SL1) (Ectothiorhodospira halophila (strain DSM 244 / SL1)) complete proteome ExPASy Proteomics Server. Swiss Institute of Bioinformatics http://hamap.expasy.org/proteomes/HALHL.html
- Zhu, Daochen; Niu, Lili; Wang, Chenxiang; Nagata, Shinichi (September 2007). "Isolation and characterisation of moderately halophilic bacteriumHalomonas ventosae DL7 synthesizing ectoine as compatible solute" (PDF). Annals of Microbiology. 57 (3): 401–406. doi:10.1007/BF03175080. S2CID 43421438.
- Gerday, C.; Glansdorff, N. (2009). EXTREMOPHILES – Volume II. Encyclopedia of life support systems. Eolss Publishers. p. 303 ff. ISBN 978-1-905839-94-0. Retrieved 18 May 2021.
- Werkhäuser, Nina; Bilstein, Andreas; Sonnemann, Uwe (1 June 2014). "Treatment of Allergic Rhinitis with Ectoine Containing Nasal Spray and Eye Drops in Comparison with Azelastine Containing Nasal Spray and Eye Drops or with Cromoglycic Acid Containing Nasal Spray". Journal of Allergy. 2014: 1–13. doi:10.1155/2014/176597. ISSN 1687-9783. PMC 4058592.
