Electroless nickel-boron plating
Electroless nickel-boron coating (often called NiB coating) is a metal plating process that can create a layer of a nickel-boron alloy on the surface of a solid substrate, like metal or plastic. The process involves dipping the substrate in a water solution containing nickel salt and a boron-containing reducing agent, such as an alkylamineborane or sodium borohydride. It is a type of electroless nickel plating. A similar process, that uses a hypophosphite as a reducing agent, yields a nickel-phosphorus coating instead.
Unlike electroplating, electroless plating processes in general not require passing an electric current through the bath and the substrate; the reduction of the metal cations in solution to metallic is achieved by purely chemical means, through an autocatalytic reaction. Thus electroless plating creates an even layer of metal regardless of the geometry of the surface – in contrast to electroplating which suffers from uneven current density due to the effect of substrate shape on the electric field at its surface. Moreover, electroless plating can be applied to non-conductive surfaces.[1]
The plating bath usually also contains buffers, complexants, and other control chemicals.
History
Electroless nickel-boron plating developed as a variant of the similar nickel-phosphorus process, discovered accidentally by Charles Adolphe Wurtz in 1844.[2]
In 1969, Harold Edward Bellis from DuPont filed a patent for a general class of electroless plating processes using sodium borohydride, dimethylamine borane, or sodium hypophosphite, in the presence of thallium salts, thus producing a metal-thallium-boron or metal-thallium-phosphorus; where the metal could be either nickel or cobalt. The boron or phosphorus contents was claimed to be variable from 0.1 to 12%, and that of thallium from 0.5 to 6%. The coatings were claimed to be "an intimate dispersion of hard trinickel boride (Ni3B) or nickel phosphide (Ni3P) in a soft matrix of nickel and thallium".[3]
Several versions of the process were patented by Charles Edward McComas in the following years:
- Bellis's formulation[3] was modified by adding stronger chelating agents ("Generation 1"). A method for applying nickel-thallium-boron to titanium without fatiguing the titanium substrate was also developed and commercialized by Purecoat International under the brand "NiBRON".[4][5]
- In 1986, McComas patented an improved formulation ("Generation 2") for nickel-cobalt-thallium-boron coating, that further increased stability and repeatability of the process. The patent claims that the coating consisted of "hard, amorphous alloy nodules of high nickel content dispersed or rooted in a softer alloy of high cobalt content".[6]
- In 1994 McComas developed another formulation ("Generation 3"), patented in 1998, that allowed consumed chemicals to be replenished during the plating, making it into a continuous process rather than a batch one.[7]
- A further improvement, patented by McComas in 2000 ("Generation 4") used lead tungstate as the stabilizer in place of thallium sulfate, at a pH of 10 to 14. This formulation has been commercialized by UCT Coatings under various brand names, including UltraCem and FailZero.[8][9]
Types
The earliest variants of electroless nickel-boron plating included thallium salts in the plating bath, and actually created nickel-thallium-boron coatings ("Type 1" in the AMS classification). Eventually formulations were devised that were free from the toxic thallium ingredients, resulting in true nickel-boron ("Type 2") coatings.[5]
Characteristics
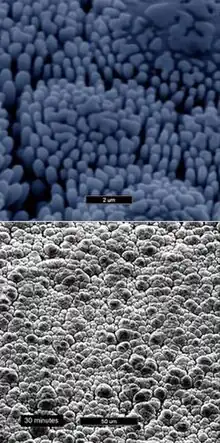
As-plated grains of amorphous nickel boron deposit in a columnar structure with the columns being perpendicular to the substrate surface and forming a nodular topography on the surface.[10] Coating will contain 2.5–8% boron by weight.[5]
The nodular structure reduces surface-to-surface contact of two mating/sliding surfaces, thus reducing friction and improving heat dissipation.[11] It is also claimed to reduces drag in both gas and liquid flows.[12]
Applications
Actual and potential applications of electroless nickel-boron coating include saw blades,[11] ship propellers,[12] down-hole crude oil pumping equipment, bushings, thrust washers and paper guide plates.
References
- ASTM B607. "Standard Specification for Autocatalytic Nickel Boron Coatings for Engineering Use- 91(2009)".
- Georgi G. Gavrilov (1979), Chemical (Electroless) Nickel-Plating. Translation by John E. Goodman. Accessed on 2018-09-08.ISBN 9780861080236
- Harold Edward Bellis (1969): "Nickel or cobalt wear-resistant compositions and coatings". US Patent 3674447. Granted on 1972-07-04, assigned to DuPont, expired on 1989-07-04
- PWA-259. "Processes and Specifications".
- SAE. "AMS 2433B-1994 Plating,nickel-thallium-boron or nickel-boron". Archived from the original on 2014-01-01. Retrieved 2013-12-31.
- Charles Edward McComas (1986): "Corrosion/wear-resistant metal alloy coating compositions". US Patent 5019163. granted on 1989-05-23, assigned to Anchor Glass, expired on 2006-05-30.
- Charles Edward McComas (1998): "Coating compositions containing nickel and boron". US Patent 6183546. Granted 2001-02-06, assigned to McComas Industries International, expired on 2018-11-02.
- "UCT Coatings, Inc".
- Charles Edward McComas (1998): "Coating compositions containing nickel and boron". US Patent 6066406. Granted 2000-05-23, assigned to Universal Chemical Technologies (UCT), expired on 2018-05-08
- Y.W. Riddle; T.O. Bailer (April 2005). "Friction and wear reduction via an Ni-B electroless bath coating for metal alloys". Journal of Materials. 57 (4): 40–45. doi:10.1007/s11837-005-0080-7.
- Charles Edward McComas and Dennis Hanlen (2008): "Coated guided pad for saw blades". US Patent Application 20090205470-A1. Filed on 2008-02-15, assigned to UCT Coatings, abandoned.
- Charles Edward McComas (2007): "Method of improving the performance of a hydrodynamic surface". US Patent Application 20090123777-A1. Filed on 2007-11-14, assigned to UCT Coatings, abandoned.