Atomic electron transition
In atomic physics and chemistry, an atomic electron transition (also called an electronic (de-)excitation, atomic transition, or quantum jump) is a change (or jump) of an electron from one energy level to another within an atom[1] or artificial atom.[2] It appears discontinuous as the electron "jumps" from one quantized energy level to another, typically in a few nanoseconds or less.
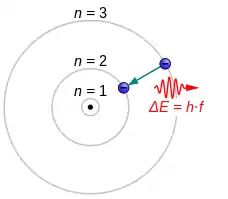
Electron transitions cause the emission or absorption of electromagnetic radiation in the form of quantized units called photons. Their statistics are Poissonian, and the time between jumps is exponentially distributed.[3] The damping time constant (which ranges from nanoseconds to a few seconds) relates to the natural, pressure, and field broadening of spectral lines. The larger the energy separation of the states between which the electron jumps, the shorter the wavelength of the photon emitted.[4] The emitted photon changes the kinetic energy of the atom, enabling the laser cooling technology to slow down the motion of atoms.
History
Danish physicist Niels Bohr first theorized that electrons can perform quantum jumps in 1913.[5] Soon after, James Franck and Gustav Ludwig Hertz proved experimentally that atoms have quantized energy states.[6]
The observability of quantum jumps was predicted by Hans Dehmelt in 1975, and they were first observed using trapped ions of barium at University of Hamburg and mercury at NIST in 1986.[4]
Recent discoveries
In 2019, it was demonstrated in an experiment with a superconducting artificial atom consisting of two strongly-hybridized transmon qubits placed inside a readout resonator cavity at 15 mK, that the evolution of some jumps is continuous, coherent, deterministic, and reversible.[7] On the other hand, other quantum jumps are inherently unpredictable.[8]
See also
References
- Schombert, James. "Quantum physics" University of Oregon Department of Physics
- Vijay, R; Slichter, D. H; Siddiqi, I (2011). "Observation of Quantum Jumps in a Superconducting Artificial Atom". Physical Review Letters. 106 (11): 110502. arXiv:1009.2969. Bibcode:2011PhRvL.106k0502V. doi:10.1103/PhysRevLett.106.110502. PMID 21469850. S2CID 35070320.
- Deléglise, S. "Observing the quantum jumps of light" (PDF). Archived from the original (PDF) on November 7, 2010. Retrieved September 17, 2010.
- Itano, W. M.; Bergquist, J. C.; Wineland, D. J. (2015). "Early observations of macroscopic quantum jumps in single atoms" (PDF). International Journal of Mass Spectrometry. 377: 403. Bibcode:2015IJMSp.377..403I. doi:10.1016/j.ijms.2014.07.005.
- Gleick, James (October 21, 1986). "PHYSICISTS FINALLY GET TO SEE QUANTUM JUMP WITH OWN EYES". The New York Times. ISSN 0362-4331. Retrieved December 6, 2021.
- "Franck-Hertz experiment | physics | Britannica". www.britannica.com. Retrieved December 6, 2021.
- Minev, Z. K.; Mundhada, S. O.; Shankar, S.; Reinhold, P.; Gutiérrez-Jáuregui, R.; Schoelkopf, R. J..; Mirrahimi, M.; Carmichael, H. J.; Devoret, M. H. (June 3, 2019). "To catch and reverse a quantum jump mid-flight". Nature. 570 (7760): 200–204. arXiv:1803.00545. Bibcode:2019Natur.570..200M. doi:10.1038/s41586-019-1287-z. PMID 31160725. S2CID 3739562.
- Snizhko, Kyrylo; Kumar, Parveen; Romito, Alessandro (September 29, 2020). "Quantum Zeno effect appears in stages". Physical Review Research. 2 (3): 033512. arXiv:2003.10476. Bibcode:2020PhRvR...2c3512S. doi:10.1103/PhysRevResearch.2.033512. S2CID 214623209.
External links
- Schrödinger, Erwin (August 1952). "Are there quantum jumps? Part I" (PDF). The British Journal for the Philosophy of Science. 3 (10): 109–123. doi:10.1093/bjps/iii.10.109. Part 2
- "There are no quantum jumps, nor are there particles!" by H. D. Zeh, Physics Letters A172, 189 (1993).
- Ball, Philip (June 5, 2019). "Quantum Leaps, Long Assumed to Be Instantaneous, Take Time". Quanta Magazine. Retrieved June 6, 2019.
- "Surface plasmon at a metal-dielectric interface with an epsilon-near-zero transition layer" by Kevin Roccapriore et al., Physical Review B 103, L161404 (2021).