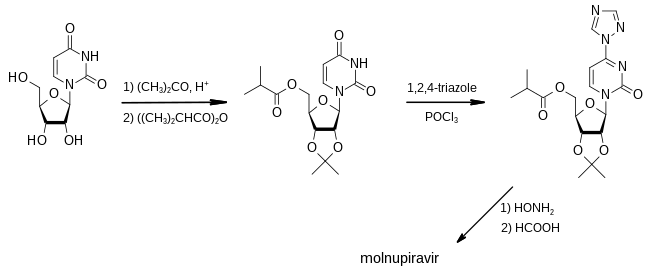
Molnupiravir
Molnupiravir, sold under the brand name Lagevrio, is an antiviral medication that inhibits the replication of certain RNA viruses.[6] It is used to treat COVID-19 in those infected by SARS-CoV-2.[6] It is taken by mouth.[6]
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌmɔːlnuˈpɪərəvɪər/ MAWL-noo-PEER-ə-veer |
| Trade names | Lagevrio |
| Other names | MK-4482, EIDD-2801 |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL |
|
| Chemical and physical data | |
| Formula | C13H19N3O7 |
| Molar mass | 329.309 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Molnupiravir is a prodrug of the synthetic nucleoside derivative N4-hydroxycytidine and exerts its antiviral action by introducing copying errors during viral RNA replication.[12][13]
Molnupiravir was originally developed to treat influenza at Emory University by the university's drug innovation company, Drug Innovation Ventures at Emory (DRIVE), but was reportedly abandoned for mutagenicity concerns.[14][15] It was then acquired by Miami-based company Ridgeback Biotherapeutics, which later partnered with Merck & Co. to develop the drug further.[16]
Based on positive results in placebo-controlled double-blind randomized clinical trials,[17][18] molnupiravir was approved for medical use in the United Kingdom in November 2021.[6][19][20][21] In December 2021, the U.S. Food and Drug Administration (FDA) granted an emergency use authorization (EUA) to molnupiravir for use in certain populations where other treatments are not feasible.[9] The emergency use authorization was only narrowly approved (13-10) because of questions about efficacy and concerns that molnupiravir's mutagenic effects could create new variants that evade immunity and prolong the COVID-19 pandemic.[22][23][24] In September 2023, molnupiravir´s mutagenicity was confirmed in a study of global SARS CoV 2 isolates after 2022: genomic changes were more common, especially where it had been used.[25]
Medical uses
Molnupiravir is indicated for the treatment of mild-to-moderate coronavirus disease (COVID-19) in adults with positive results of direct SARS-CoV-2 viral testing who are at high risk for progression to severe COVID-19[6][9] and for whom alternative FDA-authorized COVID-19 treatments are not accessible or clinically appropriate.[26] It is the second oral medication against COVID-19 after nirmatrelvir/ritonavir, but with a lower efficacy: about 30% (95% CI, 1–51%) against hospitalization or death in unvaccinated adults with mild or moderate COVID-19 and at least one risk factor for disease progression.[11]
Contraindications
Use during pregnancy is not recommended.[3] There are no human data on use during pregnancy to assess the risk of adverse maternal or fetal outcomes.[3] Based on animal data, the drug may cause fetal harm.[3]
In rats, bone and cartilage toxicity was observed after repeated dosing.[11]
Adverse effects
Adverse reactions observed in the phase III MOVe-OUT study included diarrhea (2%), nausea (1%) and dizziness (1%), all of which were mild or moderate.[11]
Overdose
The effects of overdose are unknown, treatment consists of general supportive measures such as monitoring of clinical status.[11]
Drug interactions
Based on limited available data, there are no drug interactions.[11]
Mechanism of action
Molnupiravir inhibits viral reproduction by promoting widespread mutations in the replication of viral RNA by RNA-directed RNA polymerase.[27] It is metabolized into a ribonucleoside analog that resembles cytidine, β-D-N4-hydroxycytidine 5′-triphosphate (also called EIDD-1931 5′-triphosphate or NHC-TP).[28][29][30] During replication, the virus's enzyme incorporates NHC-TP into newly made RNA instead of using real cytidine.[30]
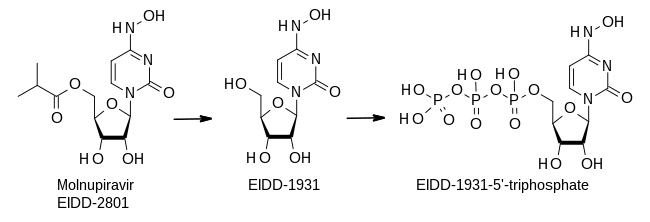
Molnupiravir can swap between two forms (tautomers), one of which mimics cytidine (C) and the other uridine (U).[31] NHC-TP is not recognized as an error by the virus's proofreading exonuclease enzymes, which can replace mutated nucleotides with corrected versions.[27] When the viral RNA polymerase attempts to copy RNA containing molnupiravir, it sometimes interprets it as C and sometimes as U. This causes more mutations in all downstream copies than the virus can survive, an effect called viral error catastrophe or lethal mutagenesis.[31]
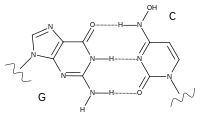
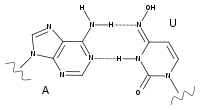
Chemistry
The first synthesis of molnupiravir was disclosed in a patent filed by Emory University in 2018.[32]
In the first step, acetone is used as a protecting group to render two of the three hydroxy groups of uridine unreactive to treatment with the acid anhydride of isobutyric acid, which converts the third hydroxy group to its ester. Treatment with 1,2,4-triazole and phosphoryl chloride produces a reactive intermediate in which the triazole portion can be replaced with hydroxylamine. Finally, removal of the protecting group using formic acid converts the material to molnupiravir.[32]: 93–95
Alternative patented routes to molnupiravir have been reviewed.[33]
History
Molnupiravir was developed at Emory University by its drug innovation company, Drug Innovation Ventures at Emory (DRIVE).[16] In 2014, DRIVE began a screening project funded by the Defense Threat Reduction Agency to find an antiviral drug targeting Venezuelan equine encephalitis virus (VEEV), which led to the discovery of EIDD-1931.[34] When turned into the prodrug EIDD-2801 (molnupiravir), the compound also showed activity against other RNA viruses including influenza, Ebola, chikungunya, and various coronaviruses.[34]
The international nonproprietary name of the drug was inspired by that of Thor's hammer, Mjölnir. The idea is that the drug will strike down the virus like a mighty blow from the god of thunder.[30]
In 2019, the National Institute of Allergy and Infectious Diseases (NIAID) approved moving molnupiravir into Phase I clinical trials for influenza.[34]
In March 2020, the research team pivoted to studying SARS-CoV-2, and successfully used molnupiravir to treat human cells infected with the novel coronavirus.[34] A study found that it is orally active against SARS-CoV-2 in ferrets.[35]
DRIVE then licensed molnupiravir for human clinical studies to Miami-based company Ridgeback Biotherapeutics, which later partnered with Merck & Co. to develop the drug further.[34][16]
The primary data supporting the U.S. Food and Drug Administration (FDA) emergency use authorization for molnupiravir are from MOVe-OUT, a randomized, double-blind, placebo-controlled clinical trial studying molnupiravir for the treatment of non-hospitalized participants with mild to moderate COVID-19 at high risk for progression to severe COVID-19 and/or hospitalization.[9][36] Participants were adults 18 and older with a pre-specified chronic medical condition or at increased risk of SARS-CoV-2 infection for other reasons who had not received a COVID-19 vaccine.[9] The main outcome measured in the trial was the percentage of people who were hospitalized or died due to any cause during 29 days of follow-up.[9] Of the 709 people who received molnupiravir, 6.8% were hospitalized or died within this period compared to 9.7% of the 699 people who received a placebo.[9]
In November 2022, the British National Institute for Health and Care Excellence decided molnupiravir should not be routinely used for Covid, as research showed it made no significant difference to hospitalization or death rates and was not cost effective. The drug was added to its "not recommended" list in draft Covid treatment guidance for consultation.[37][38]
Society and culture
Economics
In September 2021, Merck signed a voluntary licensing agreement with the Medicines Patent Pool (MPP) that allows MPP to sublicense molnupiravir and supply the COVID-19 oral medication to 105 low- and middle-income countries. The cost of the U.S. government's initial purchase was about $712 per course of treatment; treatment with generics in developing countries can cost as little as $20.[39][40]
Sales of Molnupiravir were $952 million in the fourth quarter of 2021, which in part made Merck's sales figures increase with respect to the same period of the previous year.[41]
Legal status
On 11 October 2021, Merck submitted an EUA application to the FDA, and on 30 November 2021, the FDA's Antimicrobial Drugs Advisory Committee (AMDAC) at the Center for Drug Evaluation and Research met to discuss the application.[42][43] The committee narrowly voted, 13 for and 10 opposed, to recommend authorization for adults with mild to moderate illness who are at high risk of developing severe COVID-19.[44] Concerns were expressed over the drug's low effectiveness in preventing death, which in the final trial was only 30%, as well as the increased mutation rate the drug causes, which could theoretically worsen the pandemic by driving the evolution of more dangerous variants.[44][15] In December 2021, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for molnupiravir for the treatment of mild-to-moderate coronavirus disease (COVID-19) in adults with positive results of direct SARS-CoV-2 viral testing who are at high risk for progression to severe COVID-19, including hospitalization or death, and for whom alternative COVID-19 treatment options authorized by the FDA are not accessible or clinically appropriate.[9]
In October 2021, the Committee for Medicinal Products for Human Use of the European Medicines Agency (EMA) started a rolling review of molnupiravir.[45]
In November 2021, molnupiravir was approved in the U.K. by the Medicines and Healthcare products Regulatory Agency (MHRA) for the treatment of established infections of COVID-19.[6] The MHRA issued a conditional marketing authorization applicable in the U.K., and an emergency use authorization for Northern Ireland.[6][19][46][47]
In November 2021, the Bangladesh Directorate General of Drug Administration (DGDA) authorized emergency use of molnupiravir.[48][49]
In January 2022, molnupiravir was approved for medical use in Israel.[50]
Names
Molnupiravir is the international nonproprietary name (INN).[51][52]
Generic versions are available under the brand names Molulife (Mankind) and Molena (Emcure).
Public health concerns
At a November 2021 AMDAC meeting, multiple advisors raised the concern that molnupiravir could accelerate the emergence of variants of concern.[53][54] Other scientists raised similar concerns both before and after the meeting.[55][24][56][23] These concerns were confirmed with the September 2023 publication of a study of 15 million global SARS-CoV-2 sequences: after molnupiravir had been introduced in 2022, genomic changes were more common, especially where it had been used.[25]
Research
COVID-19 clinical trial
In October 2021, preliminary results from a clinical trial (MOVe-OUT)[57][58] indicated that treatment with molnupiravir may reduce the risk of hospitalization and death from COVID-19.[59][60] The final analysis reported a 30% reduction in hospitalizations and deaths.[17][61]
Since December 2021, the PANORAMIC trial has been testing molnupiravir's effectiveness.[62][63] Results showed that for higher risk, vaccinated adults molnupiravir does not reduce the chances of hospitalisation and death. However it results in faster recovery and reduced viral load.[64][65]
In February 2023, Merck reported that the phase 3 MOVe-AHEAD trial to evaluate the safety and efficacy of Lagevrio compared to placebo in preventing the spread of SARS-CoV-2 within households did not meet its primary endpoints. With more than 1,500 participants who were free of COVID-19 and lived with someone who was recently diagnosed with the virus, patients treated with Lagevrio were 23.6% less likely than those on placebo to develop COVID after 14 days.[66][67][68]
References
- "Lagevrio APMDS". Therapeutic Goods Administration (TGA). 21 January 2022. Archived from the original on 5 February 2022. Retrieved 5 February 2022.
- "Updates to the Prescribing Medicines in Pregnancy database". Therapeutic Goods Administration (TGA). 12 May 2022. Archived from the original on 3 April 2022. Retrieved 13 May 2022.
- "TGA eBS - Product and Consumer Medicine Information Licence". Archived from the original on 5 February 2022. Retrieved 5 February 2022.
- "AusPAR: Molnupiravir". Therapeutic Goods Administration (TGA). 8 February 2022. Archived from the original on 24 March 2022. Retrieved 23 March 2022.
- Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control]. Diário Oficial da União (in Brazilian Portuguese) (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- "Summary of Product Characteristics for Lagevrio". Medicines and Healthcare products Regulatory Agency (MHRA). 4 November 2021. Archived from the original on 4 November 2021. Retrieved 4 November 2021.
- "Regulatory approval of Lagevrio (molnupiravir)". Medicines and Healthcare products Regulatory Agency (MHRA). 4 November 2021. Archived from the original on 4 November 2021. Retrieved 4 November 2021.
- "Molnupiravir capsule". DailyMed. Archived from the original on 5 January 2022. Retrieved 4 January 2022.
- "Coronavirus (COVID-19) Update: FDA Authorizes Additional Oral Antiviral for Treatment of COVID-19 in Certain Adults". U.S. Food and Drug Administration (FDA) (Press release). 23 December 2021. Archived from the original on 23 December 2021. Retrieved 23 December 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - O'Shaughnessy JA (22 March 2022). "Emergency Use Authorization 108". Letter to Merck Sharp & Dohme Corp. U.S. Food and Drug Administration (FDA).
- "Fact sheet for healthcare providers: Emergency Use Authorization for molnupiravir" (PDF). Food and Drug Administration. Merck & Co., Inc. 23 December 2021. Archived from the original on 24 December 2021.
- Toots M, Yoon JJ, Cox RM, Hart M, Sticher ZM, Makhsous N, et al. (October 2019). "Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia". Science Translational Medicine. 11 (515): eaax5866. doi:10.1126/scitranslmed.aax5866. PMC 6848974. PMID 31645453.
- Toots M, Yoon JJ, Hart M, Natchus MG, Painter GR, Plemper RK (April 2020). "Quantitative efficacy paradigms of the influenza clinical drug candidate EIDD-2801 in the ferret model". Translational Research. 218: 16–28. doi:10.1016/j.trsl.2019.12.002. PMC 7568909. PMID 31945316.
- Cohen B, Piller C (May 2020). "Emails offer look into whistleblower charges of cronyism behind potential COVID-19 drug". Science. doi:10.1126/science.abc7055.
- Cully M (January 2022). "A tale of two antiviral targets - and the COVID-19 drugs that bind them". Nature Reviews. Drug Discovery. 21 (1): 3–5. doi:10.1038/d41573-021-00202-8. PMID 34857884. S2CID 244851870.
- Aleccia J (29 September 2021). "Daily pill to treat COVID could be just months away". ABC News. Kaiser Health News. Archived from the original on 29 September 2021. Retrieved 29 September 2021.
- Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al. (December 2021). "Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients". The New England Journal of Medicine. 386 (6): 509–520. doi:10.1056/NEJMoa2116044. PMC 8693688. PMID 34914868.
- Singh AK, Singh A, Singh R, Misra A (November 2021). "Molnupiravir in COVID-19: A systematic review of literature". Diabetes & Metabolic Syndrome. 15 (6): 102329. doi:10.1016/j.dsx.2021.102329. PMC 8556684. PMID 34742052.
- "First oral antiviral for COVID-19, Lagevrio (molnupiravir), approved by MHRA" (Press release). Medicines and Healthcare products Regulatory Agency (MHRA). 4 November 2021. Archived from the original on 5 January 2022. Retrieved 4 November 2021.
- "Merck and Ridgeback's Molnupiravir, an Oral COVID-19 Antiviral Medicine, Receives First Authorization in the World". Merck (Press release). 4 November 2021. Archived from the original on 4 November 2021. Retrieved 4 November 2021.
- Robbins R, Khan AJ, Specia M (4 November 2021). "Britain Becomes First to Authorize an Antiviral Pill for Covid-19". The New York Times. Archived from the original on 28 November 2021. Retrieved 27 November 2021.
- Kimball S (30 November 2021). "FDA advisory panel narrowly endorses Merck's oral Covid treatment pill, despite reduced efficacy and safety questions". CNBC. Archived from the original on 1 January 2022. Retrieved 1 January 2022.
- Lin MZ (24 December 2021). "A new drug to treat covid could create a breeding ground for mutant viruses". The Washington Post. Archived from the original on 30 December 2021. Retrieved 1 January 2022.
- Service RF (November 2021). "A prominent virologist warns COVID-19 pill could unleash dangerous mutants. Others see little cause for alarm". Science. doi:10.1126/science.acx9591.
- Sanderson T, Hisner R, Donovan-Banfield I, Hartman H, Løchen A, Peacock TP, et al. (September 2023). "A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes". Nature: 1–3. doi:10.1038/s41586-023-06649-6. PMID 37748513. S2CID 262748823.
- Rubin R (June 2022). "From Positive to Negative to Positive Again-The Mystery of Why COVID-19 Rebounds in Some Patients Who Take Paxlovid". JAMA. 327 (24): 2380–2382. doi:10.1001/jama.2022.9925. PMID 35675094. S2CID 249465757.
- Lowe D (13 October 2021). "Molnupiravir mutations". Science (blog). Archived from the original on 21 December 2021. Retrieved 13 October 2021.
- Painter WP, Holman W, Bush JA, Almazedi F, Malik H, Eraut NC, et al. (March 2021). "Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2". Antimicrobial Agents and Chemotherapy. 65 (5). doi:10.1128/AAC.02428-20. PMC 8092915. PMID 33649113.
- Amara A, Penchala SD, Else L, Hale C, FitzGerald R, Walker L, et al. (September 2021). "The development and validation of a novel LC-MS/MS method for the simultaneous quantification of Molnupiravir and its metabolite ß-d-N4-hydroxycytidine in human plasma and saliva". Journal of Pharmaceutical and Biomedical Analysis. 206: 114356. doi:10.1016/j.jpba.2021.114356. PMC 7611757. PMID 34509661. S2CID 237493842.
- Mole B (October 2021). "Meet molnupiravir, Merck's Thor-inspired pill that hammers COVID". Ars Technica. Archived from the original on 2 October 2021. Retrieved 2 October 2021.
- Malone B, Campbell EA (September 2021). "Molnupiravir: coding for catastrophe". Nature Structural & Molecular Biology. 28 (9): 706–708. doi:10.1038/s41594-021-00657-8. PMID 34518697. S2CID 237507937.
- US application 20200276219, Painter GR, Bluemling GR, Natchus MG, Guthrie D, "N4-hydroxycytidine and derivatives and anti-viral uses related thereto", published 2020-09-03, assigned to Emory University
- Wruhs O (1986). "[Comparative study of stability following the nailing of fractures of the femur shaft. An experimental study with cadaver bones]". Wiener Klinische Wochenschrift. Supplementum (in German). 169: 3–16. PMID 3464133.
- Halford B. "An emerging antiviral takes aim at COVID-19". C&EN. Archived from the original on 2 August 2020. Retrieved 2 October 2021.
- Cox RM, Wolf JD, Plemper RK (January 2021). "Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets". Nature Microbiology. 6 (1): 11–18. doi:10.1038/s41564-020-00835-2. PMC 7755744. PMID 33273742.
- CDER Scientific Review Supporting EUA (PDF) (Report). Center for Drug Evaluation and Research. Archived from the original on 16 February 2022. Retrieved 16 February 2022.
- Donnelly L (11 February 2023). "Almost £1bn spent on anti-Covid drug that makes 'no significant difference'". The Daily Telegraph. Retrieved 13 February 2023.
- "NICE recommends 3 treatments for COVID-19 in draft guidance". National Institute for Health and Care Excellence. 16 November 2022. Retrieved 13 February 2023.
- "The Medicines Patent Pool (MPP) and Merck Enter Into License Agreement for Molnupiravir, an Investigational Oral Antiviral COVID-19 Medicine, to Increase Broad Access in Low- and Middle-Income Countries". Merck (Press release). Archived from the original on 27 October 2021. Retrieved 28 October 2021.
- "Merck Will Share Formula for Its Covid Pill With Poor Countries". The New York Times. 27 October 2021. Archived from the original on 27 November 2021. Retrieved 27 November 2021.
- Erman M (3 February 2022). "Merck sees 2022 sales up nearly 20%, mostly on molnupiravir". Reuters. Retrieved 29 October 2022.
- "Merck and Ridgeback Announce Submission of Emergency Use Authorization Application to the U.S. FDA for Molnupiravir, an Investigational Oral Antiviral Medicine, for the Treatment of Mild-to-Moderate COVID-19 in At Risk Adults". Merck (Press release). Archived from the original on 17 October 2021. Retrieved 17 October 2021.
- "FDA to Hold Advisory Committee Meeting to Discuss Merck and Ridgeback's EUA Application for COVID-19 Oral Treatment". U.S. Food and Drug Administration (FDA) (Press release). 18 October 2021. Archived from the original on 18 October 2021. Retrieved 19 October 2021.
- Hensley S (30 November 2021). "An FDA panel supports Merck COVID drug in mixed vote". NPR. Archived from the original on 4 January 2022. Retrieved 3 December 2021.
- "COVID-19: EMA starts rolling review of molnupiravir". European Medicines Agency (EMA). 25 October 2021. Archived from the original on 4 November 2021. Retrieved 6 November 2021.
- Reed J (4 November 2021). "First pill to treat Covid gets approval in UK". BBC News Online. Archived from the original on 4 November 2021. Retrieved 4 November 2021.
{{cite web}}: CS1 maint: overridden setting (link) - Whipple T (4 November 2021). "UK first to approve 'game-changing' antiviral Covid pill". The Times. Archived from the original on 4 November 2021. Retrieved 5 November 2021.
{{cite news}}: CS1 maint: overridden setting (link) - "Oral medicine for Covid-19 now available in Bangladesh". The Business Standard. 9 November 2021. Archived from the original on 10 November 2021. Retrieved 10 November 2021.
- "Eskayef's Covid pill hits market". The Daily Star. 10 November 2021. Archived from the original on 9 November 2021. Retrieved 10 November 2021.
- "The Anti-Viral Drug Lagevrio (Molnupiravir) for the Treatment of COVID-19 Has Been Approved". Archived from the original on 4 July 2022. Retrieved 30 June 2022.
- World Health Organization (2021). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 85" (PDF). WHO Drug Information. 35 (1). Archived (PDF) from the original on 19 April 2021. Retrieved 23 December 2021.
- World Health Organization (2022). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 87" (PDF). WHO Drug Information. 36 (1). Archived (PDF) from the original on 1 June 2022. Retrieved 24 April 2022.
- Kimball S (30 November 2021). "FDA advisory panel narrowly endorses Merck's oral Covid treatment pill, despite reduced efficacy and safety questions". CNBC. Archived from the original on 1 January 2022. Retrieved 4 January 2022.
- Walker M (30 November 2021). "FDA Panel Narrowly Backs Merck's COVID Pill". MedPage Today. Archived from the original on 4 January 2022. Retrieved 4 January 2022.
- Nelson CW, Otto SP (29 November 2021). "Mutagenic antivirals: the evolutionary risk of low doses". Virological. Archived from the original on 1 January 2022. Retrieved 4 January 2022.
- Lovett S (11 December 2021). "Scientists' caution over use of new antiviral pill in immunosuppressed". The Independent. Archived from the original on 4 January 2022. Retrieved 4 January 2022.
- "Merck and Ridgeback Biotherapeutics Provide Update on Progress of Clinical Development Program for Molnupiravir, an Investigational Oral Therapeutic for the Treatment of Mild-to-Moderate COVID-19". Merck (Press release). 15 April 2021. Archived from the original on 28 November 2021. Retrieved 28 November 2021.
- Clinical trial number NCT04575597 for "Efficacy and Safety of Molnupiravir (MK-4482) in Non-Hospitalized Adult Participants With COVID-19 (MK-4482-002)" at ClinicalTrials.gov
- "Merck and Ridgeback's Investigational Oral Antiviral Molnupiravir Reduced the Risk of Hospitalization or Death by Approximately 50 Percent Compared to Placebo for Patients with Mild or Moderate COVID-19 in Positive Interim Analysis of Phase 3 Study". Merck (Press release). 1 October 2021. Archived from the original on 1 October 2021. Retrieved 28 November 2021.
- Herper M (1 October 2021). "Merck's antiviral pill reduces hospitalization of Covid patients, a possible game-changer for treatment". Stat. Archived from the original on 1 October 2021. Retrieved 2 October 2021.
- Mishra M, Erman M (26 November 2021). "Merck's COVID-19 pill significantly less effective in new analysis". Reuters. Archived from the original on 1 December 2021. Retrieved 2 December 2021.
- "NIHR funds community COVID-19 antiviral trial". NIHR. Archived from the original on 16 March 2022. Retrieved 16 March 2022.
- "Thousands needed to try a new Covid antiviral treatment". BBC News. 25 January 2022. Archived from the original on 16 March 2022. Retrieved 16 March 2022.
- Butler CC, Hobbs FD, Gbinigie OA, Rahman NM, Hayward G, Richards DB, et al. (December 2022). "Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial". Lancet. 401 (10373): 281–293. doi:10.1016/S0140-6736(22)02597-1. PMC 9779781. PMID 36566761.
- "Pill for Covid does not reduce risk of hospitalisation or death, UK study finds". the Guardian. 22 December 2022. Retrieved 29 December 2022.
- Merck Sharp & Dohme LLC (5 December 2022). "A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Efficacy and Safety of MK-4482 for the Prevention of COVID-19 (Laboratory-confirmed SARS-CoV-2 Infection With Symptoms) in Adults Residing With a Person With COVID-19". Clinicaltrials.
- "Merck Provides Update on Phase 3 MOVe-AHEAD Trial Evaluating LAGEVRIO™ (molnupiravir) for Post-exposure Prophylaxis for Prevention of COVID-19". Merck.com. Retrieved 22 February 2023.
- "Merck's COVID pill Lagevrio comes up short in household COVID exposure study". Fierce Pharma. 21 February 2023. Retrieved 22 February 2023.
Further reading
- Thorlund K, Sheldrick K, Meyerowitz-Katz G, Singh S, Hill A (March 2022). "Making Statistical Sense of the Molnupiravir MOVe-OUT Clinical Trial". Am J Trop Med Hyg. 106 (5): 1301–1304. doi:10.4269/ajtmh.21-1339. PMC 9128711. PMID 35276667. S2CID 247406958.