Emricasan
Emricasan (IDN-6556, PF-03491390) is a potential drug invented in 1998 by Idun Pharmaceuticals.[1][2] The drug was acquired by Pfizer in 2005[3] and then sold to Conatus Pharmaceuticals in 2010.[1] Conatus in turn licensed emricasan to Novartis in 2017 for exclusive development and commercialization.[4]
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
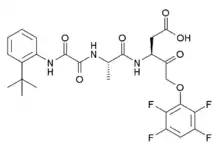
| Formula | C26H27F4N3O7 |
| Molar mass | 569.501 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
The substance acts as a pan-caspase inhibitor and has antiapoptotic and antiinflammatory effects.[4] It was developed for the treatment of liver disease[5] and has been granted fast track designation by the FDA for the treatment of non-alcoholic steatohepatitis cirrhosis[6][7][8] The substance is the first pan-caspase inhibitor to advance to broad clinical testing, and its novel mechanism of action has led to research using it for other potential applications.[9][10]
References
- McCallister E (Aug 9, 2010). "IDUN IT AGAIN: Conatus Reacquires Idun Assets From Pfizer, Hopes Data Will Attract Investors". BioCentury.
- Linton SD, Aja T, Armstrong RA, Bai X, Chen LS, Chen N, et al. (November 2005). "First-in-class pan caspase inhibitor developed for the treatment of liver disease". Journal of Medicinal Chemistry. 48 (22): 6779–82. doi:10.1021/jm050307e. PMID 16250635.
- "Pfizer to acquire Idun Pharmaceuticals in cash transaction". Scrip. May 2005.
- Taylor P (May 4, 2017). "Novartis adds to NASH pipeline, activating license for Conatus' emricasan". FierceBiotech.
- Hoglen NC, Chen LS, Fisher CD, Hirakawa BP, Groessl T, Contreras PC (May 2004). "Characterization of IDN-6556 (3-[2-(2-tert-butyl-phenylaminooxalyl)-amino]-propionylamino]-4-oxo-5-(2,3,5,6-tetrafluoro-phenoxy)-pentanoic acid): a liver-targeted caspase inhibitor". The Journal of Pharmacology and Experimental Therapeutics. 309 (2): 634–40. doi:10.1124/jpet.103.062034. PMID 14742742.
- Marriott N (10 May 2017). "Lonza NEWS Novartis enter agreement with Conatus for NASH treatment". European Pharmaceutical Review.
- Haddad JJ (September 2013). "Current opinion on 3-[2-[(2-tert-butyl-phenylaminooxalyl)-amino]-propionylamino]- 4-oxo-5-(2,3,5,6-tetrafluoro-phenoxy)-pentanoic acid, an investigational drug targeting caspases and caspase-like proteases: the clinical trials in sight and recent anti-inflammatory advances". Recent Patents on Inflammation & Allergy Drug Discovery. 7 (3): 229–58. doi:10.2174/1872213X113079990017. PMID 23859695.
- Rotman Y, Sanyal AJ (January 2017). "Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease". Gut. 66 (1): 180–190. doi:10.1136/gutjnl-2016-312431. PMID 27646933. S2CID 3461795.
- Brumatti G, Ma C, Lalaoui N, Nguyen NY, Navarro M, Tanzer MC, et al. (May 2016). "The caspase-8 inhibitor emricasan combines with the SMAC mimetic birinapant to induce necroptosis and treat acute myeloid leukemia". Science Translational Medicine. 8 (339): 339ra69. doi:10.1126/scitranslmed.aad3099. PMID 27194727.
- Xu M, Lee EM, Wen Z, Cheng Y, Huang WK, Qian X, et al. (October 2016). "Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen". Nature Medicine. 22 (10): 1101–1107. doi:10.1038/nm.4184. PMC 5386783. PMID 27571349.