Chiral analysis
Chiral analysis refers to the quantification of component enantiomers of racemic drug substances or pharmaceutical compounds. Other synonyms commonly used include enantiomer analysis, enantiomeric analysis, and enantioselective analysis. Chiral analysis includes all analytical procedures focused on the characterization of the properties of chiral drugs.[1] Chiral analysis is usually performed with chiral separation methods where the enantiomers are separated on an analytical scale and simultaneously assayed for each enantiomer.[2][3][4][5][6][7][8][9][10]
Many compounds of biological and pharmacological interest are chiral. Pharmacodynamic, pharmacokinetic, and toxicological properties of the enantiomers of racemic chiral drugs has expanded significantly and become a key issue for both the pharmaceutical industry and regulatory agencies.[11][12][13][14][15][16] Typically one of the enantiomers is more active pharmacologically (eutomer). In several cases, unwanted side effects or even toxic effects may occur with the inactive enantiomer (distomer).[17] Even if the side effects are not that serious, the inactive enantiomer has to be metabolized, this puts an unnecessary burden on the already stressed out system of the patient. Large differences in activity between enantiomers reveal the need to accurate assessment of enantiomeric purity of pharmaceutical, agrochemicals, and other chemical entities like fragrances and flavors become very important. Moreover, the moment a racemic therapeutic is placed in a biological system, a chiral environment, it is no more 50:50 due enantioselective absorption, distribution, metabolism, and elimination (ADME) process. Hence to track the individual enantiomeric profile there is a need for chiral analysis tool.
Chiral technology is an active subject matter related to asymmetric synthesis[18] and enantioselective analysis, particularly in the area of chiral chromatography. As a consequence of the advances in chiral technology, a number of pharmaceuticals currently marketed as racemic drugs are undergoing re-assessment as chiral specific products or chiral switches.[19][20][21][22] Despite the choice to foster either a single enantiomer or racemic drug, in the current regulatory environment, there will be a need for enantioselective investigations. This poses a big challenge to pharmaceutical analysts and chromatographers involved in drug development process. In pharmaceutical research and development stereochemical analytical methodology may be required to comprehend enantioselective drug action and disposition, chiral purity assessment, study stereochemical stability during formulation and production, assess dosage forms, enantiospecific bioavailability and bioequivalence investigations of chiral drugs. Besides pharmaceutical applications chiral analysis[23] plays a major role in the study of biological and environmental samples and also in the forensic field.[24] Chiral analysis methods and applications between the period 2010 and 2020 are exhaustively reviewed recently.[25] There are number of articles, columns, and interviews in LCGC relating to emerging trends in chiral analysis and its application in drug discovery and development process.[26][27][28][29][30][31]
For chiral examination there is a need to have the right chiral environment. This could be provided as a plane polarized light, an additional chiral compound or by exploiting the inborn chirality of nature. The chiral analytical strategies incorporate physical, biological, and separation science techniques. Recently an optical-based absolute chiral analysis has been reported.[32] The most frequently employed technique in enantioselective analysis involve the separation science techniques, in particular chiral chromatographic methods or chiral chromatography. Today wide range of CSPs are available commercially based on various chiral selectors including polysaccharides, cyclodextrins, glycopeptide antibiotics, proteins, Pirkle, crown ethers, etc. to achieve analysis of chiral molecules.[33]
Chiral chromatography
This term has become very popular and commonly used in practice. But the appropriate expression is "enantioselective chromatography".[34] Chiral chromatography has advanced to turn into the most preferred technique for the determination of enantiomeric purity as well as separation of pure enantiomers both on analytical and preparative scale. Chiral chromatographic assay is the first step in any study pertaining to enantioselective synthesis or separation. This includes the use of techniques viz. gas chromatography (GC), high performance liquid chromatography (HPLC), chiral supercritical fluid chromatography (SFC), capillary electrophoresis (CE)[35] and thin-layer chromatography (TLC).[36][37][38][39][40] The result of a literature survey done identifies HPLC-based chiral assays as the most dominating technology in use.[41] An overview of various analytical methods engaged for chiral separation and analysis are listed in the table.[42][43][44]
| Method | Brief narrative of principle and application |
|---|---|
| Chromatographic | |
| Chiral HPLC | Chiral HPLC is used to separate enantiomers either by direct or indirect separation mode. Widely employed to check enantiomeric purity, provided the reference standards of the racemate or the two enantiomers are available. Capable of distinguishing between enantiomers and from the racemate; (+) from (-) and (±) |
| Chiral GC | Majority of chiral separations using GC are done with cyclodextrin derivatives as chiral selector. This method can be used to distinguish between enantiomers and from the racemate; (+) from (-) and (±) |
| Supercritical fluid chromatography (SFC) | Principle is very similar to that of HPLC. But SFC typically uses carbon dioxide as the mobile phase. Hence there is a need to pressurize the entire chromatographic flow path. SFC can differentiate enantiomers and enantiomer from the racemate; (+) from (-) and (±) |
| Chiral capillary electrophoresis (CE)[45] | Chiral CE is based largely on separation of enantiomers by complex formation with cyclodextrins which is used as the chiral selector. Able to differentiate between enantiomers and from the racemate; (+) from (-) and (±) |
| Spectroscopic | |
| Polarimetry | Polarimetry uses the innate property of chiral molecules to rotate the plane-polarized light in equal and opposite direction. This method can be used to distinguish between enantiomers and from the racemate; (+) from (-) and (±) |
| Optical rotatory dispersion (ORD) | ORD is a curve obtained by plotting the measured optical activity of a chiral compound as a function of the wavelength of the light used. Distinguish between enantiomers and from the racemate; (+) from (-) and (±) |
Circular dichroism
(CD) |
CD measures the differential absorption of left and right circularly polarized light by a chiral compound. These chiroptical techniques can be employed to identify and/or determine enantiomers |
| Nuclear magnetic resonance (NMR) | NMR spectroscopy done using chiral shift reagents or chiral solvating reagents. Capable of discriminating enantiomers as well as the racemate |
| Infrared (IR) | Differentiate the racemate and its enantiomers but not between the enantiomeric pair; (+) or (-) from (±) |
| Calorimetry | |
| Differential scanning calorimetry (DSC) | The underlying principle is to measure the energy absorbed or evolved by a sample as a function of temperature. Data can distinguish between an enantiomer and the racemate, but not one enantiomer from its mirror-mage |
Principle - separation of enantiomers
In an isotopic/achiral environment, enantiomers exhibit identical physicochemical properties, and therefore are indistinguishable under these conditions. For the separation of chiral molecules the challenge is to construct the right chiral environment. In a chromatographic system there are three variables namely, the chiral analyte (CA), mobile phase and stationary phase, that can be manipulated to provide the crucial chiral environment. The strategy is to make these variables to interact with a chiral auxiliary (chiral selector, CS) whereby it forms a diastereomeric complex which has different physicochemical properties and makes it possible to separate the enantiomers. Based on the nature of the diastereomeric complex formed between the CS-CA species, enantiomer separation mythologies are categorized as indirect and direct enantiomer separation mode
Indirect separation of enantiomer
Indirect enantiomer separation involves the interaction between the chiral analyte (CA) of interest and the suitable reactive CS (in this case it is an enantiopure chiral derivatizing agent, CDA) leading to the formation of a covalent diastereomeric complex that can be separated with an achiral chromatographic technique. Therapeutic agents often contain reactive functional groups (amino, hydroxyl, epoxy, carbonyl and carboxylic acid, etc.) in their structures. They are converted into covalently bonded diastereomeric derivatives using enantiomerically pure chiral derivatizing agent. The diastereomers thus formed unlike enantiomers, exhibit different physicochemical properties in an achiral environment and are eventually separated as a result of differential retention time on a stationary phase.[46][47][48][49][50] The success of this approach depends on the availability of stable enantiopure chiral derivatizing agent (CDA) and on the presence of a suitable reactive functional group in the chiral drug molecule for covalent formation of diastereomeric derivative. The reaction of a racemic, (R,S)- Drug with a chirally and chemically pure chiral derivatizing agent, (R’)-CDA, will afford diastereomeric products, (R)-Drug-(R')-CDA + (S)-Drug-(R’)- CDA. The chiral derivatization reaction scheme is illustrated in the box on the right hand side.
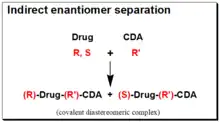
In contrast to enantiomers, diastereomers have different physicochemical properties that make them separable on regular achiral stationary phases. The major benefit of the indirect methodology is that conventional achiral stationary phase/mobile phase system may be used for the separation of the generated diastereomers. Thus, considerable flexibility in chromatographic conditions is available to achieve the desired separation and to eliminate interferences from metabolites and endogenous substances. Moreover, the sensitivity of the method can be enhanced by sensible choice of the CDA and the chromatographic detection system. But this indirect approach to enantiomeric analysis has some potential problems. These include availability of a suitable functional group on the enantiomer for derivatization, enantiomeric purity of the CDA, racemization of the CDA during derivatization, and racemization of the analyte during the derivatization. Currently, however, the application of indirect analytical approaches is in decline.
Direct separation of enantiomers
Direct enantiomer separation involves the formation of a transient rather than covalent diastereomeric complexation between the chiral selector/discriminator and the analyte (drug enantiomer). In this approach, the subtle energy differences between the reversibly formed noncovalent diastereomeric complexes are exploited for chiral recognition. The direct chromatographic enantiomer separation may be achieved in two different ways, the chiral mobile phase additive and chiral stationary phase mode.[51]
Chiral mobile phase additive (CMPA)
In this approach, an enantiomerically pure compound, the chiral selector, is added to the mobile phase and separation happens on a conventional achiral column. When a mixture of enantiomers is introduced into the chromatographic system, the individual enantiomers form transient diastereomeric complexes with the chiral mobile phase additive. In the chiral mobile phase additive technique, two possible mechanisms may operate: one possibility is that CMPA and the enantiomers may form diastereomers in the mobile phase. Another is that the stationary phase may be coated with the CMPA, leading to diastereomeric interactions with the enantiomeric pairs during chromatographic separation process. It is observed that both the mechanisms may happen depending on the characteristic of the stationary phase and mobile phase employed.[52] Of late this method finds limited application.
Chiral stationary phase (CSP)
In the direct enantiomer separation the most popular approach is use of chiral stationary phases. In this case the site of the chiral selector is on the stationary phase. Stationary phase consist of an inert solid support (usually silica microparticles) on to the surface of which a single enantiomer of a chiral molecule (selector) is either coated/adsorbed or chemically linked and that forms the chiral stationary phase. Commonly used chiral selectors include polysaccharides, proteins, cyclodextrins, etc. An interesting review of chiral stationary phase development and application in chiral analysis appeared in LCGC magazine, 2011.[53]
Chiral recognition
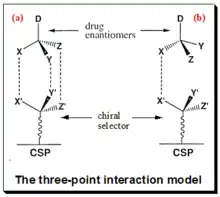
Chiral recognition implies the ability of chiral stationery phases to interact differently with mirror-image molecules, leading to their separation. The mechanism of enantiomeric resolution using CSPs is generally attributed to the “three-point" interaction model (fig.1.) between the analyte and the chiral selector in the stationary phase. Also known as the Dalgliesh model.[54] Under this model, for chiral recognition, and hence enantiomeric resolution to happen on a CSP one of the enantiomers of the analyte must be involved in three simultaneous interactions. This means to say the one of enantiomers is able to have a good interaction with the complimentary sites on the chiral selector attached to the CSP. While Its mirror-image partner may only interact at two or one such sites. In the figure, enantiomer (a), has the correct configuration of the ligands (X, Y and Z) for three-point interactions with the complimentary sites (X’, Y’ and Z’) on the CSP, while its mirror image (b) can only interact at one site. The dotted lines (-----) indicate interaction with complimentary sites.
The diastereomeric complexes thus formed will have different energies of interaction. The enantiomer forming the more stable complex will have less energy and stay longer in the stationary phase compared to the less stable complex with higher energy. The success of chiral separation basically depends in manipulating the subtle energy differences between the reversibly formed non-covalent transient diastereomeric complexes. The energy difference reflects the magnitude of enantioselectivity. Mobile phase has a major role in stabilizing the diastereomeric complex and thus in chiral separation. This simplified bimolecular interaction model is a treatment suitable for theoretical purposes. Mobile phase plays a key role in chiral recognition mechanism. Components of MP (such as bulk solvents, modifiers, buffer salts, additives) not only influence the conformational flexibility of CS and CA molecules but also their degree of ionization. The types of interaction involved in the analyte-selector interaction vary depending on the nature of the CSP used. These may include hydrogen bonding, dipole-dipole, π-π, electrostatic, hydrophobic or steric interactions, and inclusion complex formation.
Classical chiral selectors and CSPs
The intense research for development of efficient chiral selectors has resulted in the synthesis of over 1400 CSPs and over 200 CSPs have been commercialized and available in the market.[55] The most commonly employed chiral selectors are categorized and presented in the table.
| Type of CSP | Chemistry | Chiral distinction mechanism | Loading capacity[56]
mg/g (CSP) |
| Polymer | Polysaccharides | H-bonding, dipole-dipole interactions; inclusion ion complexes also play an important role | 5-150 |
| Proteins | Hydrophobic and electrostatic interactions | 0.1 - 0.2 | |
| Macrocycles | Native and
derivatized Cyclodextrins |
Inclusion complexes, H-bonding; solute enters the cavities within the CSP to form inclusion complexes | 0.1 - 0.5 |
| Glycopeptide antibiotics | H-bonding; π-π interactions, dipole sacking; steric; hydrophobic pocket | 0.1 - 0.5 | |
| Crown ethers | 0.1 - 0.5 | ||
| Low-molecular weight
scaffolds |
Pirkle type | H-bonding; π-π interactions, dipole sacking | 1-50 |
| Ligand-exchange | Coordination complexes to metals | 0.1 - 0.5 |
Background
It is surprising to note that In 1980, there was no single chiral stationary phase available in the market for performing chiral chromatography. However, In late 1980s the subject of enantioselective chromatography attracted growing interest, particularly under the drive of the institution of Okamoto in Japan, the teams of Pirkle, and Armstrong in the US, Schurig and König in Germany, Lindner in Austria, and Francotte in Switzerland .[57] The Polysaccharides, amylose and cellulose, form the most abundant chiral polymers on earth. These naturally occurring polysaccharides form basis for an important class of chiral selectors.
Chemistry
Amylose and cellulose cannot be used as such due to poor resolution and difficulty in handling. But the carbamate and benzoate derivatives of these polymers, especially amylose and cellulose, demonstrate excellent properties as chiral selectors for chromatographic separation. A large number of polysaccharide-based CSPs are commercially available for chiral separation. These CSPs showed tremendous chiral recognition capability to resolve a wide range of chiral analytes. Many of these CSPs have been marketed by Daicel Chemical Industries, Ltd., and some of the popular ones are listed in the table.
| # | Adsorbent / Stationary phase description | Chiral stationary phase (Trade name) |
| 1. | Cellulose tris-(3,5-dimethyl phenyl carbamate) | Chiralcel OD® (Daicel) |
| 2. | Cellulose tris-(4-methyl benzoate ) | Chiralcel OJ® / Lux® Cellulose-3 (Phenomenex) |
| 3. | Amylose tris(3,5-dimethyl phenyl carbamate) | Chiralpak AD® (Daicel) |
| 4. | Amylose tris(S)-a-methyl benzyl carbamate | Chiralpak AS® (Daicel) |
| 5. | Cellulose tris(3-chloro-4-methylphenylcarbamate) | Chiralcel OZ® (Daicel) |
| 6. | Amylose tris(5-chloro-2-methylphenylcarbamate) | Chiralcel AY® (Daicel) |
| 7. | Amylose tris(3-chloro-4-methylphenylcarbamate) | Chirlpak AZ® (Daicel) |
| 8. | Cellulose tris(4-chloro-3-methylphenylcarbamate) | Chiralcel OX® (Daicel) |
| 9. | tris (3,5-dimethylphenyl) carbamoyl cellulose selector | RegisCell® |
| 10. | tris (3,5-dimethylphenyl) carbamoyl amylose selector | RegisPack® |
These CSPs are compatible with NP/RP and SFC and also used for analytical, semi-preparative and preparative separations. Many screening research studies conducted at different labs go to suggest that the four CSPs namely Chiralcel OD, Chiralcel OJ, Chiralpak AD, and Chiralpak As are capable of resolving more than 80% of the chiral separations due to their adaptability and high loading capacity.[58][59][60] These four polysaccharide chiral stationary stationary phases are referred to as the "golden four".[61]
Polysaccharide CSPs are prepared with high quality silica support on to which the polymeric chiral selector (amylose/cellulose dr.) is physically coated (coated CSP) or chemically immobilized (immobilized CSP). Separations can be done in normal phase, reversed-phase, and polar organic mode. While working with coated polysaccharide CSP solvent selection should be done with caution. One should not use drastic solvents such as dichloromethane, chloroform, toluene, ethyl acetate, THF; 1,4-dioxane; acetone; DMSO, etc. These so called "non-standard" solvents will dissolve the silica and irreversibly destroy the stationary phase. The limited resistance of these coated phases to many solvents lead to the development of immobilized polysaccharide CSP. The table below presents some of the immobilized CSP commercially available and with the alternates wherever accessible.[62][63]
| # | Chiral selector/Adsorbent chemistry | Chiral stationary phase/ Suppliers | ||
|---|---|---|---|---|
| Daicel® | Phenomenex® | Reprosil® | ||
| 1 | Amylose tris(3,5-dimethylphenyl)carbamate (as in Chiralpak® AD) | Chiralpak® IA | Lux® i-Amylose-1 | Reprosil® MIA |
| 2 | Cellulose tris(3,5-dimethylphenyl)carbamate (as in Chiralcel® OD) | Chiralcel® IB | ----- | Reprosil® MIB |
| 3 | Cellulose tris(3,5-dichlorophenyl)carbamate | Chiralcel® IC | Lux® i-Cellulose-5 | Reprosil® MIC |
| 4 | Amylose tris(3-chlorophenyl)carbamate | Chiralpak® ID | ----- | ----- |
| 5 | Amylose tris(3,5-dichlorophenyl)carbamate | Chiralpak® IE | ----- | ----- |
| 6 | Amylose tris(3-chloro,4-methylphenyl)carbamate | Chiralpak® IF | ----- | ----- |
| 7 | Amylose tris(3-chloro,5-methylphenyl)carbamate | Chiralpak® IG | ----- | ----- |
These immobilized CSP are much more rugged and the "non-standard" solvents can be employed. Thus expanding the choice of co-solvent. The major strength of immobilized CSPs are high solvent versatility in selection of mobile phase composition, enhanced sample solubility, high selectivity, robustness and extended durability, excellent column efficiency, and broad application domain in the resolution of enantiomers. Solvent is a key factor in HPLC MD. More solvents to play with means better sample solubility, Improves resolution, and enables effective chiral method development.
Mechanism
Number of chiral environments are created within the polymer. Cavities are formed between adjacent glucose units, and spaces/channels between polysaccharide chains. These chiral cavities or channels give the chiral discrimination capability to polysaccharide CSPs. The mechanism of Chiral discrimination is not well understood but believed to involve hydrogen bonding and dipole-dipole interaction between the analyte molecule and the ester or carbamate linkage of the CSP.
Application
Some of the applications of these CSPs include the direct chiral analysis of β-adrenergic blockers such as metoprolol[64] and celiprolol,[65] the calcium channel blocker, felodipine[66] and the anticonvulsant agent, ethotoin.[67]
Macrocyclic CSPs
An interesting way of achieving chiral distinction on a CSP is the use of selectors with chiral cavity. These chiral selectors are attached to the stationary phase support material. In this category, there are basically three types of cavity chiral selectors namely cyclodextrins,[68] crown ethers[69] and macrocyclic glycopeptide antibiotics.[70] Among these cyclodextrin based CSP is popular. In this type of CSPs the enantioselective guest-host interaction governs the chiral distinction.
Cyclodextrin-type CSP
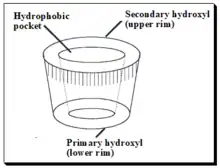
Cyclodextrins (CDs) are cyclic oligosaccharides of six, seven, or eight glucose units designated as α, β, and γ cyclodextrins respectively. Depicted in the diagram below. Daniel Armstrong is considered the pioneer of micelle and cyclodextrin-based separations. Cyclodextrins are covalently attached to silica by Armstrong process and provide stable CSPs.[71] The primary hydroxyl groups are used to anchor the CD molecules to the modified silica surface. CDs are chiral because of innate chirality of the building blocks, glucose units. In cyclodextrin the glucose units are α-(1,4)- connected. The shape of CD looks like a shortened cone (see the sketch). The inner surface of the cone forms moderately hydrophobic pocket. The width of the CD-cavity is identified with the quantity of glucose units present. In cyclodextrins, secondary hydroxyl groups (OH-2 and - 3) line the upper rim of the cavity, and an essential 6-hydroxyl group is positioned at the lower rim. The hydroxyl group offer chiral binding points, which appear to be fundamental for enantioselectivity. Apolar glyosidic oxygen makes the pit hydrophobic and guarantees inclusion complexing of the hydrophobic moiety of analytes. Interactions between the polar area of an analyte and secondary hydroxyl groups at the mouth of the pit, joined with the hydrophobic connections inside the pit, give a unique two-point fit and lead to enantioselectivity.
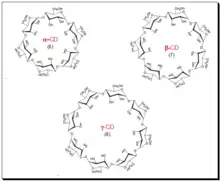
Selectivity of a cyclodextrin phase is dependent on two key factors namely the size and structure of the analyte since it is based on a simple fit-unfit geometric criteria. An aromatic ring or cycloalkyl ring should be attached near the stereogenic center of the analyte. Substituents at or near the analyte chiral center must be able to interact with the hydroxyl groups at the entrance of the CD cavity through H-bonding.[72] α-Cyclodextrin holds small aromatic molecules, whereas β-cyclodextrin incorporates both naphthyl groups and substituted phenyl groups. The aqueous compatibility of CD and its unique molecular structure make the CD- bonded phase highly suitable for use in chiral HPLC analysis of drugs. One further benefit of CD is that they are generally less expensive than the other CSPs. Some of the major shortcomings of CD CSPs is that it is limited to compounds that can enter into CD cavity, minor structural changes in analyte leads to unpredictable effect on resolution, often poor efficiency and cannot invert elution order.
Enantiomers of propranolol, metoprolol, chlorpheniramine, verapamil, hexobarbitaI, methadone and much more drugs have been separated using immobilized β-cyclodextrin.[73]
Initially natural CDs have been used as the chiral selector. Later, modified cyclodextrin structures have been prepared by derivatizing the secondary hydroxyl groups present on the CD molecule.[74][75] Incorporation of these additional functional groups may improve the chiral recognition capability by possibly modifying the chiral pocket and creating extra auxiliary interaction site. This approach enabled to expand the range of target chiral analytes that could be separated. A number of chiral pharmaceuticals has been resolved using derivatized CDs including ibuprofen, suprofen, flurbiprofen from NSAID category and b-blockers like metoprolol and atenolol.[76] A brief list of cyclodextrin-based chiral stationary stationary phases available in the market is furnished in the table below.[77]
| Chiral stationary phase (Brand name) | Chiral selector/chemical description | Mode # | Company/ Major distributor |
| Cyclobond® I 2000 | Natural β-Cyclodextrin | RP, PO | Advanced Separation Technology (Astec), Whippany, NJ |
| Cyclobond® II | Natural γ-Cyclodextrin | RP, PO | Astec |
| Cyclobond® III | Natural α-Cyclodextrin | RP, PO | Astec |
| Cyclobond® I 2000 AC | Acetylated β-Cyclodextrin | RP, PO | Astec |
| Cyclobond® I 2000 SP | (S)-Hydroxypropyl β-Cyclodextrin | RP, PO | Astec |
| Cyclobond® I 2000 SN | (S)-1(1-napthyl)ethyl carbamoyl-β-Cyclodextrin | RP, NP, PO | Astec |
| Cyclobond® I 2000 RN | (R)-1(1-napthyl)ethyl carbamoyl-β-Cyclodextrin | RP, NP, PO | Astec |
| Cyclobond® I 2000 DMP | 3,5-dimethylphenyl carbamoyl-β-Cyclodextrin | RP, NP, PO | Astec |
| Cyclobond® II AC | Acetylated γ-Cyclodextrin | RP, PO | Astec |
| Cyclobond® III AC | Acetylated α-Cyclodextrin | RP, PO | Astec |
| ChiraDex® | Native α-Cyclodextrin | RP, PO | Merck, Germany |
| ChiraDex® Gamma | Native γ-Cyclodextrin | RP, PO | Merck |
| Note: # RP, reversed phase; PO, Polar organic; NP, normal phase. | |||
Glycopeptide-type CSP
Armstrong introduced macrocyclic glycopeptides (also known as glycopeptide antibiotics) as a new class of chiral selector for liquid chromatography in 1994.[78] At present, vancomycin, teicoplanin and ristocetin are available under the brand names Chirobiotic V, Chirobiotic T and Chirobiotic R respectively. These cyclic glycopeptides have multiple chiral centers and a cup-like inclusion area to which a floating sugar lid is attached. Similar to protein chiral selectors, the amphoteric cyclic glycopeptides consist of peptide and carbohydrate binding sites leading to possibilities for different modes of interaction beside the formation of inclusion complexation. In this chiral selector the cavities are shallower than that of CDs and hence the interactions are weaker, allows more rapid solute exchange between phases, higher column efficiency. operates in normal phase, reversed-phase and polar organic phase.
The complex structural nature of glycopeptide antibiotic class of CSP has made the understanding of the mechanism of chiral recognition at molecular level tricky. For instance, vancomycin molecule has 18 stereogenic centers in the molecule and offers a complex cyclodextrin-like chiral environment. In comparison to a single basket of cyclodextrins, vancomycin consists of three baskets, resulting in a more complex inclusion of appropriate guest molecules. The attractive forces include π-π interactions, hydrogen bonding, ionic interactions, and dipole stacking. A carboxylic acid and a secondary amine group located on the rim of the cup and can participate in ionic interactions. Vancomycin stationary phases operate in reversed, normal and polar organic phase modes.
Wide range of chiral analysis has been done using chirobiotic CSPs.[79] The antihypertensive drugs viz. oxprenolol, pindolol, propranolol have been separated using vancomycin and teicoplanin chirobiotic CSPS. The NSAID drugs ketoprofen and ibuprofen has been separated using ristocetin CSP.
Crown ether-type CSP
Crown ethers, like cyclodextrin-type CSPs contain a chiral cavity. Crown ethers are immobilized on the silica surface to form chiral stationary phase. Crown ethers contain oxygen atoms within the cavity. The cyclic structure that contains apolar ethylene groups between oxygen forms hydrophobic inner cavity. Cram et al., introduced CSP based on chiral crown ethers and accomplished separation of amino acid.[80] The crucial chiral recognition principle underlying crown ether-based enantiomer separation is based on the formation of numerous hydrogen bonds between the protonated primary amino group of the analyte and the ether oxygens of the crown structure.[81] This structural requirement confines the application of crown ether-type CSPs to chiral compounds having primary amino groups adjoining the chiral centers, such as amino acids, amino acid derivatives. Progress in the field of crown ether-type CSPs have been reviewed.[82]
Protein-type CSP
Proteins are complex, high-molecular weight biopolymers. They are inherently chiral being composed of L-amino acids and possess ordered 3D-structure. They are known to bind/interact stereoselectively with small molecules reversibly, making them extremely versatile CSPs for chiral separation of drug molecules. Hermansson made use of this property to develop number of CSPs by immobilizing proteins on to silica surface.[83] They operate under reverse phase mode (phosphate buffer and organic modifiers).
Protein polymer remains in twisted form because of the different intramolecular bonding. These bonding create different type of chiral loops/grooves present in the protein molecule. Separation mechanism of proteins depends on unique combination of hydrophobic and polar interactions by which the analytes are oriented to chiral surfaces. H-bonding and charge transfer may also contribute to enantioselectivity. The mechanism of chiral distinction by proteins is mostly not well established due to their complex nature. Several proteins based CSP have been employed for chiral drug analysis including α-acid glycoprotein (enantiopac; chiral-AGP), ovomucoid protein (Ultron ES DVM), human serum albumin (HSA).[84] α-AGP CSP (chiral AGP), has been employed for the quantification of atenolol enantiomers in biological matrices,[85] for pharmacokinetic investigation of racemic metoprolol.[86] The major weakness of protein based CSPs include low loading capacity, protein phases are expensive, extremely fragile, delicate to handle, very low column efficiency, cannot invert elution order.
Pirkle-type CSP
Pirkle and co-workers pioneered the development of a variety of CSPs based on charge-transfer complexation and simultaneous hydrogen bonding.[87][88][89] These phases are also referred to as Brush-type CSPs. The Pirkle phases are based on aromatic π-acid (3,5-dinitrobenzoyI ring) and π- basic (naphthalene) derivative. In addition to π-π interaction sites, they have hydrogen-bonding and dipole-dipole interaction sites provided by an amide, urea or ester functionality. Strong three-point interaction, according to Dalgleish's model, enables enantioseparation. These phases are classified into π-electron-acceptor, π-electron-donor or π-electron acceptor-donor phase.
A number of Pirkle-type CSPs are commercially available. They are used most often in the normal phase mode. The ionic form of the DNPBG (3,5-dinitrobenzoyl-phenylglycine) CSP has been successfully employed to achieve separation of racemic propranolol in biological fluid. Many compounds of pharmaceutical interest including enantiomers of naproxen and metoprolol has been separated using Pirkle CSP.[90][91]
Novel chiral selectors and CSPs
During the last couple of years there has been developments of CSPs based on novel chiral selectors viz. chitosan derivatives, cylofructan derivatives[92] and chiral porous materials for HPLC chiral separation.[93]
Chitosan derivatives based CSP
Cyclofructan derivatives based CSP
Chiral porous materials based CSP
See also
References
- Chiral Analysis. Elsevier. 2018. doi:10.1016/c2017-0-00050-2. ISBN 978-0-444-64027-7.
- Chen, LiZhu; Zhu, DeQiu; Xiang, Ping (2021). "Recent advances in chiral analysis for biosamples in clinical research and forensic toxicology". Bioanalysis. 13 (6): 493–511. doi:10.4155/bio-2020-0330. ISSN 1757-6180. PMID 33719527. S2CID 232229593.
- Chiral analysis. Kenneth W. Busch, Marianna A. Busch. Amsterdam: Elsevier. 2006. ISBN 978-0-444-51669-5. OCLC 162580325.
{{cite book}}: CS1 maint: others (link) - Williams, Reed C; Edwards, Janet F; Joshi, Amita S; Aubry, Ann-Francoise (2001). "Chiral analysis of drug substance in clinical plasma extracts using achiral HPLC with circular dichroism detection". Journal of Pharmaceutical and Biomedical Analysis. 25 (3–4): 501–509. doi:10.1016/s0731-7085(00)00527-6. ISSN 0731-7085. PMID 11377030.
- Porter, W. H. (1991-01-01). "Resolution of chiral drugs". Pure and Applied Chemistry. 63 (8): 1119–1122. doi:10.1351/pac199163081119. ISSN 1365-3075. S2CID 35860450.
- Wozniak, Timothy J.; Bopp, Ronald J.; Jensen, Eric C. (1991). "Chiral drugs: An industrial analytical perspective". Journal of Pharmaceutical and Biomedical Analysis. 9 (5): 363–382. doi:10.1016/0731-7085(91)80160-b. ISSN 0731-7085. PMID 1932271.
- Doyle, Thomas D (1991). "Analytical criteria for chiral High-performance liquid chromatography". In Ahuja, Satinder (ed.). Chiral separations by liquid chromatography. USA: American Chemical Society. pp. 27–42. ISBN 0-8412-2116-2.
- Pasutto, Franco M. (1992). "Mirror Images: The Analysis of Pharmaceutical Enantiomers". The Journal of Clinical Pharmacology. 32 (10): 917–924. doi:10.1002/j.1552-4604.1992.tb04639.x. ISSN 0091-2700. PMID 1447399. S2CID 34481858.
- Cancelliere, Giovanna; D’Acquarica, Ilaria; Gasparrini, Francesco; Misiti, Domenico; Villani, Claudio (1999). "Synthesis and applications of novel, highly efficient HPLC chiral stationary phases: a chiral dimension in drug research analysis". Pharmaceutical Science & Technology Today. 2 (12): 484–492. doi:10.1016/s1461-5347(99)00218-7. ISSN 1461-5347. PMID 10603466.
- "Gurus of Chiral Separations". The Analytical Scientist. Retrieved 2023-01-17.
- Francotte, Eric; Lindner, Wolfgang (2006). Chirality in drug research. Eric Francotte, W. Lindner. Weinheim: Wiley-VCH. p. 205. ISBN 978-3-527-60943-7. OCLC 163578005.
- Ariëns, E. J. (1984). "Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology". European Journal of Clinical Pharmacology. 26 (6): 663–668. doi:10.1007/bf00541922. ISSN 0031-6970. PMID 6092093. S2CID 30916093.
- Jamali, F.; Mehvar, R.; Pasutto, F.M. (1989). "Enantioselective Aspects of Drug Action and Disposition: Therapeutic Pitfalls". Journal of Pharmaceutical Sciences. 78 (9): 695–715. doi:10.1002/jps.2600780902. ISSN 0022-3549. PMID 2685226.
- Weissinger, Judi (1989). "Considerations in the Development of Stereoisomeric Drugs: FDA Viewpoint". Drug Information Journal. 23 (4): 663–667. doi:10.1177/009286158902300420. ISSN 0092-8615. S2CID 72571037.
- Gross, M (1991). "Development of chiral drugs in an evolving regulatory environment". Regulatory Affairs. 3: 483–493.
- De Camp, Wilson H. (1989). "Letter to the editor". Chirality. 1 (2): 97–98. doi:10.1002/chir.530010202. ISSN 0899-0042. PMID 2642047.
- Ariëns, Everardus J. (1986). "Stereochemistry: A source of problems in medicinal chemistry". Medicinal Research Reviews. 6 (4): 451–466. doi:10.1002/med.2610060404. ISSN 0198-6325. PMID 3534485. S2CID 36115871.
- Sheldon, Roger, A (1993). Chirotechnology - Industrial synthesis of optically active compounds. New York: Marcel Dekker, Inc., New York. pp. 73–382. ISBN 0-8247-9143-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Agranat, Israel; Caner, Hava; Caldwell, John (2002). "Putting chirality to work: the strategy of chiral switches". Nature Reviews Drug Discovery. 1 (10): 753–768. doi:10.1038/nrd915. ISSN 1474-1776. PMID 12360254. S2CID 1543301.
- Nowak, Richard (2003). "Single-isomer levalbuterol: A review of the acute data". Current Allergy and Asthma Reports. 3 (2): 172–178. doi:10.1007/s11882-003-0031-8. ISSN 1529-7322. PMID 12562558. S2CID 46090018.
- Tucker, Geoffrey T (2000). "Chiral switches". The Lancet. 355 (9209): 1085–1087. doi:10.1016/s0140-6736(00)02047-x. ISSN 0140-6736. PMID 10744105. S2CID 30715334.
- Gristwood, Robert W. (2002). "Cardiac and CNS Toxicity of Levobupivacaine". Drug Safety. 25 (3): 153–163. doi:10.2165/00002018-200225030-00002. ISSN 0114-5916. PMID 11945112. S2CID 71466303.
- Iacob, Bogdan-Cezar (2015). Chiral analysis of beta-blockers (1. Aufl ed.). Saarbrücken. ISBN 978-3-659-64269-2. OCLC 1185782844.
{{cite book}}: CS1 maint: location missing publisher (link) - Ribeiro, Cláudia; Santos, Cristiana; Gonçalves, Valter; Ramos, Ana; Afonso, Carlos; Tiritan, Maria (2018-01-28). "Chiral Drug Analysis in Forensic Chemistry: An Overview". Molecules. 23 (2): 262. doi:10.3390/molecules23020262. ISSN 1420-3049. PMC 6017579. PMID 29382109.
- Chen, LiZhu; Zhu, DeQiu; Xiang, Ping (2021). "Recent advances in chiral analysis for biosamples in clinical research and forensic toxicology". Bioanalysis. 13 (6): 493–511. doi:10.4155/bio-2020-0330. ISSN 1757-6180. PMID 33719527. S2CID 232229593.
- Leslie S. Ettre, Emanuel Gil-Av and the Separation of Enantiomers on Chiral Stationary Phases by Chromatography, LCGC North America-04-01-2007, Volume 25, Issue 4, Pages: 382–395
- Claudio Brunelli, Supercritical Fluid Chromatography in the Pharmaceutical Industry: Implementation in Development and Quality Control, LC GC, Special Issues, Special Issues-10-02-2018, Volume 31, Issue 10, Pages: 40–46
- Kate Mosford, Chiral Chromatography in antiepileptic drug development and epilepsy therapy, LC GC, The Column-04-16-2018, Volume 14, Issue 4, April 16, 2018
- Current Trends in chiral chromatography, LC GC, The Column-04-08-2014, Volume 10, Issue 6, April 8, 2014.
- Emerging Trends in Pharmaceutical Analysis, LC GC, E-Separation Solutions-11-25-2014, Volume 0, Issue 0
- Francotte, Eric (October 1, 2016). "Contemporary Analysis of Chiral Molecules". LC GC Magazine. 29 (10–03–2016): 31–37.
- Bougas, Lykourgos; Byron, Joseph; Budker, Dmitry; Williams, Jonathan (2022-06-03). "Absolute optical chiral analysis using cavity-enhanced polarimetry". Science Advances. 8 (22): eabm3749. Bibcode:2022SciA....8M3749B. doi:10.1126/sciadv.abm3749. ISSN 2375-2548. PMC 9166628. PMID 35658039.
- Eric Francotte, Contemporary Analysis of Chiral Molecules, LC GC, Special Issue-10-03-2016, Volume 29, Issue 10, Pages: 31–37
- Eliel, Ernest L. (1997). <428::aid-chir5>3.0.co;2-1 "Infelicitous stereochemical nomenclature". Chirality. 9 (5–6): 428–430. doi:10.1002/(sici)1520-636x(1997)9:5/6<428::aid-chir5>3.0.co;2-1. ISSN 0899-0042.
- Chankvetadze, Bezhan (1997). Capillary electrophoresis in chiral analysis. Chichester: John Wiley & Sons, USA. ISBN 0-585-26760-X.
- Beesley, Thomas E; P.W. Scott, Raymond (2001-05-30). John Wiley & Sons, Ltd (ed.). Chiral chromatography. USA: Wiley. doi:10.1002/047001590x. ISBN 978-0-470-01617-6.
- Allenmark, S. G (1988). "Chromatographic enantioseparation : methods and applications". Flavour and Fragrance Journal. Chichester: Ellis Horwood, Chichester. 4 (1): 45. doi:10.1002/ffj.2730040111.
- Snyder, L.R; Kirkland, J.J.; Glajch (1997). Practical HPLC method development (2nd ed.). Wiley-Interscience: J.L. pp. 537–613. ISBN 0-471-00703-X.
- Souter, RW (1985). Chromatographic Separation of Stereoisomers. Florida: CRC Press, Boca Raton.
- Zief, M; Crane, L.J., eds. (1988). Chromatographic Chiral Separations. New York: Marcel Dekker, New York.
- Maier, Norbert M; Linder, Wolfgang (2006). Francotte, Eric; Linder, Wolfgang (eds.). Chirality in drug research. Germany: Wiley-VCH Verlag GmbH & Co. pp. 189–260. ISBN 3-527-31076-2.
- Ahuja, Satinder (2011). Chiral separation methods for pharmaceutical and biotechnology products. New Jersey: John Wiley & Sons, Inc., New Jersey. ISBN 978-0-470-40691-5.
- Wozniak, Timothy J.; Bopp, Ronald J.; Jensen, Eric C. (1991). "Chiral drugs: An industrial analytical perspective". Journal of Pharmaceutical and Biomedical Analysis. 9 (5): 363–382. doi:10.1016/0731-7085(91)80160-b. ISSN 0731-7085. PMID 1932271.
- Yanan He, Chiral analysis in drug discovery, Innovations in Pharmaceutical Technology, (magazine), 19-23, December, 2010
- Chankvetadze, Bezhan (1997). Capillary electrophoresis in chiral analysis. Chichester: John Wiley. ISBN 0-585-26760-X. OCLC 45729067.
- Lunn, G; Hellwig, L.C (1998). Handbook of Derivatization Reaction for HPLC. New York: Wiley- lnterscience, New York. ISBN 978-0-471-23889-8.
- Linder, W (1988). "Indirect separation of enantiomers by liquid chromatography". Chromatographic Science Series. 40: 91–130.
- Sun, Xian Xiang; Sun, Ling Zhi; Aboul-Enein, Hassan Y. (2001). "Chiral derivatization reagents for drug enantioseparation by high-performance liquid chromatography based upon pre-column derivatization and formation of diastereomers: enantioselectivity and related structure". Biomedical Chromatography. 15 (2): 116–132. doi:10.1002/bmc.41. ISSN 0269-3879. PMID 11268052.
- Srinivas, Nuggehally R. (2004). "Evaluation of experimental strategies for the development of chiral chromatographic methods based on diastereomer formation". Biomedical Chromatography. 18 (4): 207–233. doi:10.1002/bmc.352. ISSN 0269-3879. PMID 15162384.
- Haginaka, Jun (2002). "Pharmaceutical and biomedical applications of enantioseparations using liquid chromatographic techniques". Journal of Pharmaceutical and Biomedical Analysis. 27 (3–4): 357–372. doi:10.1016/s0731-7085(01)00652-5. ISSN 0731-7085. PMID 11755739.
- Snyder, Lloyd R. (1997). Practical HPLC method development. J. J. Kirkland, Joseph L. Glajch (2nd ed.). New York: Wiley. pp. 537–613. ISBN 0-585-30111-5.
- Pettersson, C (1989). Krustulovic, A.M. (ed.). Chiral separations by HPLC applications to pharmaceutical compound. Chinchester: Ellis Horwood, Chichester. pp. 124–146.
- Thomas E. Beesley, Review of Chiral Stationary Phase Development and Chiral Applications, LCGC Europe, 05-01-2011, Volume 24, Issue 5, Pages: 270–276
- Dalgliesh, C. E. (1952). "756. The optical resolution of aromatic amino-acids on paper chromatograms". Journal of the Chemical Society (Resumed): 3940–3942. doi:10.1039/jr9520003940. ISSN 0368-1769.
- Chirality in drug research. Eric Francotte, W. Lindner. Weinheim: Wiley-VCH. 2006. p. 205. ISBN 978-3-527-60943-7. OCLC 163578005.
{{cite book}}: CS1 maint: others (link) - Zhang, Yingru; Wu, Dauh-Rurng; Wang-Iverson, David B.; Tymiak, Adrienne A. (2005). "Enantioselective chromatography in drug discovery". Drug Discovery Today. 10 (8): 571–577. doi:10.1016/s1359-6446(05)03407-0. ISSN 1359-6446. PMID 15837600.
- Francotte, Eric R. (2017-08-09). "Polysaccharide Derivatives as Unique Chiral Selectors for Enantioselective Chromatography". CHIMIA International Journal for Chemistry. 71 (7): 430–450. doi:10.2533/chimia.2017.430. ISSN 0009-4293. PMID 28779767.
- Borman, Phil; Boughtflower, Bob; Cattanach, Kaye; Crane, Kathy; Freebairn, Keith; Jonas, Greg; Mutton, Ian; Patel, Asha; Sanders, Matt; Thompson, Duncan (2003). "Comparative performances of selected chiral HPLC, SFC, and CE systems with a chemically diverse sample set". Chirality. 15 (S1): S1–S12. doi:10.1002/chir.10260. ISSN 0899-0042. PMID 12884369.
- Perrin, C; Matthijs, N; Mangelings, D; Granier-Loyaux, C; Maftouh, M; Massart, D.L; Vander Heyden, Y (2002). "Screening approach for chiral separation of pharmaceuticals". Journal of Chromatography A. 966 (1–2): 119–134. doi:10.1016/s0021-9673(02)00746-x. ISSN 0021-9673. PMID 12214686.
- Norbert M. Maier and Wolfgang Linder (2006). Chirality in drug research. Eric Francotte, W. Lindner. Weinheim: Wiley-VCH. p. 209. ISBN 3-527-31076-2. OCLC 163578005.
- Speybrouck, David; Lipka, Emmanuelle (2016). "Preparative supercritical fluid chromatography: A powerful tool for chiral separations". Journal of Chromatography A. 1467: 33–55. doi:10.1016/j.chroma.2016.07.050. PMID 27524302.
- Product lists, Daicel chiral technologies, Inc. 2020.
- "Polysaccharide chiral columns". 2021.
- Straka, Robert J.; Johnson, Kjel A.; Marshall, Peter S.; Remmel, Rory P. (1990). "Analysis of metoprolol enantiomers in human serum by liquid chromatography on a cellulose-based chiral stationary phase". Journal of Chromatography B: Biomedical Sciences and Applications. 530 (1): 83–93. doi:10.1016/s0378-4347(00)82305-1. ISSN 0378-4347. PMID 2277122.
- Hartmann, C.; Krauss, D.; Spahn, H.; Mutschler, E. (1989). "Simultaneous determination of (R)- and (S)-celiprolol in human plasma and urine: High-performance liquid chromatographic assay on a chiral stationary phase with fluorimetric detection". Journal of Chromatography B: Biomedical Sciences and Applications. 496 (2): 387–396. doi:10.1016/s0378-4347(00)82586-4. ISSN 0378-4347. PMID 2575621.
- Soons, P.A.; Roosemalen, M.C.M.; Breimer, D.D. (1990). "Enantioselective determination of felodipine and other chiral dihydropyridine calcium entry blockers in human plasma". Journal of Chromatography B: Biomedical Sciences and Applications. 528 (2): 343–356. doi:10.1016/s0378-4347(00)82393-2. ISSN 0378-4347. PMID 2384574.
- Inotsume, Nobuo; Fujii, Junko; Honda, Mikiko; Nakano, Masahiro; Higashi, Akimasa; Matsuda, Ichiro (1988). "Stereoselective analysis of the enantiomers of ethotoin in human serum using chiral stationary phase liquid chromatography and gas chromatography—mass spectrometry". Journal of Chromatography B: Biomedical Sciences and Applications. 428 (2): 402–407. doi:10.1016/s0378-4347(00)83935-3. ISSN 0378-4347. PMID 2905704.
- Cyclobond Handbook. Astec, Whippany, New Jersey. 1992.
- Shinbo, Toshio; Yamaguchi, Tomohiko; Nishimura, Koichiro; Sugiura, Masaaki (1987). "Chromatographic separation of racemic amino acids by use of chiral crown ether-coated reversed-phase packings". Journal of Chromatography A. 405: 145–153. doi:10.1016/s0021-9673(01)81756-8. ISSN 0021-9673. PMID 3693463.
- Armstrong, Daniel W.; Tang, Yubing.; Chen, Shushi.; Zhou, Yiwen.; Bagwill, Christina.; Chen, Jing-Ran. (1994-04-01). "Macrocyclic Antibiotics as a New Class of Chiral Selectors for Liquid Chromatography". Analytical Chemistry. 66 (9): 1473–1484. doi:10.1021/ac00081a019. ISSN 0003-2700.
- Ward, TJ and Armstrong, DW (1986). "Improved cyclodextrin chiral phases: A comparison and review". J. Liq. Chromatogr. 9 (2–3): 407–423. doi:10.1080/01483918608076644.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Cyclobond Handbook. New Jersey: Astec, Whippany. 1992.
- Armstrong, D.; Ward, T.; Armstrong, R.; Beesley, T. (1986-05-30). "Separation of drug stereoisomers by the formation of beta-cyclodextrin inclusion complexes". Science. 232 (4754): 1132–1135. Bibcode:1986Sci...232.1132A. doi:10.1126/science.3704640. ISSN 0036-8075. PMID 3704640.
- Amstrong, D.W; Chang, D.V; Lee, S.H. (1991). "(R)-and (S)-Naphthylethylcarbamate-substituted β-cyclo-dextrin bonded stationary phases for the reversed-phase liquid chromatographic separation of enantiomers". J. Chromatogr. 539: 83–90. doi:10.1016/S0021-9673(01)95362-2.
- User's Guide for Chiral Phase Columns. J.T. Baker, Inc. Phillipsburg, NJ. 1992.
- Armstrong, Daniel W.; Zhang, Bo (2001). "Product Review: Chiral Stationary Phases for HPLC". Analytical Chemistry. 73 (19): 557 A–561 A. doi:10.1021/ac012526n. ISSN 0003-2700.
- Mitchell, Clifford R.; Armstrong, Daniel W. (2004), "Cyclodextrin-Based Chiral Stationary Phases for Liquid Chromatography: A Twenty-Year Overview ", Chiral Separations, New Jersey: Humana Press, vol. 243, p. 68, doi:10.1385/1-59259-648-7:061, ISBN 1-58829-150-2, PMID 14970618
- Armstrong, Daniel W.; Tang, Yubing.; Chen, Shushi.; Zhou, Yiwen.; Bagwill, Christina.; Chen, Jing-Ran. (1994-04-01). "Macrocyclic Antibiotics as a New Class of Chiral Selectors for Liquid Chromatography". Analytical Chemistry. 66 (9): 1473–1484. doi:10.1021/ac00081a019. ISSN 0003-2700.
- Gübitz, Gerald; Schmid, Martin G (2004). "Enantiomeric Separations by HPLC Using Macrocyclic Glycopeptide-Based Chiral Stationary Phases: An Overview". In Tom Ling Xiao and Daniel W. Armstrong (ed.). Chiral Separations Methods and Protocols. New Jersey: Human Press, New Jersey. pp. 113–171. ISBN 1-58829-150-2.
- Sousa, Lynn R.; Sogah, G. D. Y.; Hoffman, Dale H.; Cram, Donald J. (1978). "Host-guest complexation. 12. Total optical resolution of amine and amino ester salts by chromatography". Journal of the American Chemical Society. 100 (14): 4569–4576. doi:10.1021/ja00482a041. ISSN 0002-7863.
- Machida, Yoshio; Nishi, Hiroyuki; Nakamura, Kouji (1999). <173::aid-chir1>3.0.co;2-p "Crystallographic studies for the chiral recognition of the novel chiral stationary phase derived from (+)-(R)-18-crown-6 tetracarboxylic acid". Chirality. 11 (3): 173–178. doi:10.1002/(sici)1520-636x(1999)11:3<173::aid-chir1>3.0.co;2-p. ISSN 0899-0042.
- Hyun, Myung Ho (2003-03-01). "Characterization of liquid chromatographic chiral separation on chiral crown ether stationary phases". Journal of Separation Science. 26 (3–4): 242–250. doi:10.1002/jssc.200390030. ISSN 1615-9306.
- Hermansson, Jörgen (1983). "Direct liquid chromatographic resolution of racemic drugs using α1-acid glycoprotein as the chiral stationary phase". Journal of Chromatography A. 269: 71–80. doi:10.1016/s0021-9673(01)90787-3. ISSN 0021-9673.
- Narayanan, Sunanda R. (1992). "Immobilized proteins as chromatographic supports for chiral resolution". Journal of Pharmaceutical and Biomedical Analysis. 10 (4): 251–262. doi:10.1016/0731-7085(92)80037-n. ISSN 0731-7085. PMID 1420455.
- Enquist, M; Hermansson, J (1989). "Separation and quantitation of (R)- and (S)-atenolol in human plasma and urine using an ?1-AGP column". Chirality. 1 (3): 209–215. doi:10.1002/chir.530010306. ISSN 0899-0042. PMID 2642050.
- Persson, B.-A.; Balme´r, K.; Lagerstro¨m, P.-O.; Schill, G. (1990). "Enantioselective determination of metoprolol in plasma by liquid chromatography on a silica-bonded α 1-acid glycoprotein column". Journal of Chromatography A. 500: 629–636. doi:10.1016/s0021-9673(00)96097-7. ISSN 0021-9673. PMID 2329154.
- Pirkle, W. H.; House, D. W. (1979). "Chiral high-performance liquid chromatographic stationary phases. 1. Separation of the enantiomers of sulfoxides, amines, amino acids, alcohols, hydroxy acids, lactones, and mercaptans". The Journal of Organic Chemistry. 44 (12): 1957–1960. doi:10.1021/jo01326a014. ISSN 0022-3263.
- Pirkle, W.H.; Mahler, George S.; Pochapsky, Thomas C.; Hyun, Myung Ho (1987). "Direct chromatographic separation of enantiomeric diol derivatives". Journal of Chromatography A. 388: 307–314. doi:10.1016/s0021-9673(01)94492-9. ISSN 0021-9673.
- Pirkle, WH; Pochapsky, TC (1989). "Considerations of chiral recognitions relevant to the liquid chromatographic separation of enantiomers". Chem. Rev. 89 (2): 347–362. doi:10.1021/cr00092a006.
- Pirkle, W.H.; Burke, J.A. (1991). "Chiral stationary phase designed for β-blockers". Journal of Chromatography A. 557 (1–2): 173–185. doi:10.1016/s0021-9673(01)87131-4. ISSN 0021-9673. PMID 1683876.
- Regis Chemical Company brochure, Regis Technologies, Inc. Morton Grove, IL. 1993.
- Aboul‐Enein, Hassan Y.; Kannappan, Valliappan; Kanthiah, Selvakumar (2022). "Impact of cyclofructan derivatives as efficient chiral selector in chiral analysis: An overview". Chirality. 34 (2): 364–373. doi:10.1002/chir.23396. ISSN 0899-0042. PMID 34806232. S2CID 244523210.
- Xie, Sheng-Ming; Yuan, Li-Ming (2018-09-12). "Recent development trends for chiral stationary phases based on chitosan derivatives, cyclofructan derivatives and chiral porous materials in high performance liquid chromatography". Journal of Separation Science. 42 (1): 6–20. doi:10.1002/jssc.201800656. ISSN 1615-9306. PMID 30152091. S2CID 52098380.