Endochondral ossification
Endochondral ossification[1][2] is one of the two essential processes during fetal development of the mammalian skeletal system by which bone tissue is produced. Unlike intramembranous ossification, the other process by which bone tissue is produced, cartilage is present during endochondral ossification. Endochondral ossification is also an essential process during the rudimentary formation of long bones,[3] the growth of the length of long bones,[4] and the natural healing of bone fractures.[5]
| Endochondral ossification | |
|---|---|
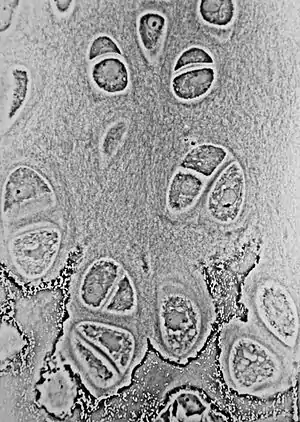 Light micrograph of undecalcified epiphyseal plate showing endochondral ossification: healthy chondrocytes (top) become degenerating ones (bottom), characteristically displaying a calcified extracellular matrix. | |
| Anatomical terminology |
Growth of the cartilage model
The cartilage model will grow in length by continuous cell division of chondrocytes, which is accompanied by further secretion of extracellular matrix. This is called interstitial growth. The process of appositional growth occurs when the cartilage model also grows in thickness due to the addition of more extracellular matrix on the peripheral cartilage surface, which is accompanied by new chondroblasts that develop from the perichondrium.
Primary center of ossification
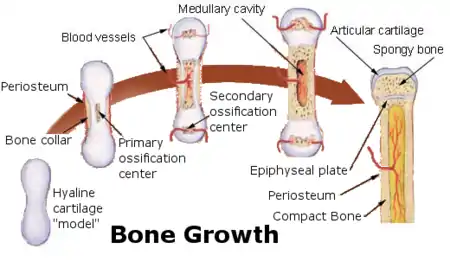
The first site of ossification occurs in the primary center of ossification, which is in the middle of diaphysis (shaft). Then:
- Formation of periosteum
- The perichondrium becomes the periosteum. The periosteum contains a layer of undifferentiated cells (osteoprogenitor cells) which later become osteoblasts.
- Formation of bone collar
- The osteoblasts secrete osteoid against the shaft of the cartilage model (Appositional Growth). This serves as support for the new bone.
- Calcification of matrix
- Chondrocytes in the primary center of ossification begin to grow (hypertrophy). They stop secreting collagen and other proteoglycans and begin secreting alkaline phosphatase, an enzyme essential for mineral deposition. Then calcification of the matrix occurs and osteoprogenitor cells that entered the cavity via the periosteal bud, use the calcified matrix as a scaffold and begin to secrete osteoid, which forms the bone trabecula. Osteoclasts, formed from macrophages, break down spongy bone to form the medullary (bone marrow) cavity.
Secondary center of ossification
About the time of birth in mammals, a secondary ossification center appears in each end (epiphysis) of long bones. Periosteal buds carry mesenchyme and blood vessels in and the process is similar to that occurring in a primary ossification center. The cartilage between the primary and secondary ossification centers is called the epiphyseal plate, and it continues to form new cartilage, which is replaced by bone, a process that results in an increase in length of the bone. Growth continues until the individual is about 20 years old or until the cartilage in the plate is replaced by bone. The point of union of the primary and secondary ossification centers is called the epiphyseal line.
Appositional bone growth
The growth in diameter of bones around the diaphysis occurs by deposition of bone beneath the periosteum. Osteoclasts in the interior cavity continue to resorb bone until its ultimate thickness is achieved, at which point the rate of formation on the outside and degradation from the inside is constant.
Histology
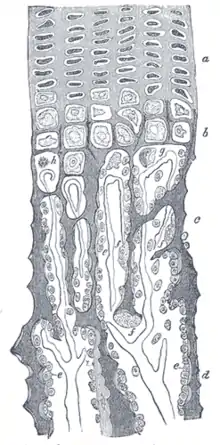
During endochondral ossification, five distinct zones can be seen at the light-microscope level.
| Name | Definition |
|---|---|
| Zone of resting cartilage | This zone contains normal, resting hyaline cartilage. |
| Zone of proliferation / cell columns | In this zone, chondrocytes undergo rapid mitosis, forming distinctive looking stacks. |
| Zone of maturation / hypertrophy | In this zone, the chondrocytes undergo hypertrophy (become enlarged). Chondrocytes contain large amounts of glycogen and begin to secrete alkaline phosphatase. |
| Zone of calcification | In this zone, chondrocytes are either dying or dead, leaving cavities that will later become invaded by bone-forming cells. Chondrocytes here die when they can no longer receive nutrients or eliminate wastes via diffusion. This is because the calcified matrix is much less hydrated than hyaline cartilage. |
| Zone of ossification | Osteoprogenitor cells invade the area and differentiate into osteoblasts, which elaborate matrix that becomes calcified on the surface of calcified cartilage. This is followed by resorption of the calcified cartilage/calcified bone complex. |
Fracture healing
During fracture healing, cartilage is often formed and is called callus. This cartilage ultimately develops into new bone tissue through the process of endochondral ossification. Recently it has been shown that biomimetic bone like apatite inhibits formation of bone through endochondral ossification pathway via hyperstimulation of extracellular calcium sensing receptor (CaSR).[6]
Examples in human body
Tubular and flat bones, vertebrae, the skull base, ethmoids, and the ends of the clavicles are formed by endochondral ossification.[7]
Additional images
 Masson Goldner trichrome stain of growth plate in a rabbit tibia.
Masson Goldner trichrome stain of growth plate in a rabbit tibia.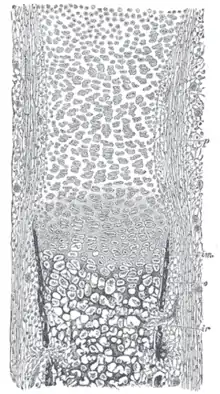 Section of fetal bone of cat. ir. Irruption of the subperiosteal tissue. p. Fibrous layer of the periosteum. o. Layer of osteoblasts. im. Subperiosteal bony deposit. (From Quain’s “Anatomy,” E. A. Schäfer.)
Section of fetal bone of cat. ir. Irruption of the subperiosteal tissue. p. Fibrous layer of the periosteum. o. Layer of osteoblasts. im. Subperiosteal bony deposit. (From Quain’s “Anatomy,” E. A. Schäfer.)
See also
References
- Etymology from Greek: ἔνδον/endon, "within", and χόνδρος/chondros, "cartilage"
- "Etymology of the English word endochondral". myEtymology.
- Netter, Frank H. (1987), Musculoskeletal system: anatomy, physiology, and metabolic disorders. Summit, New Jersey: Ciba-Geigy Corporation ISBN 0-914168-88-6, p. 130: One exception is the clavicle.
- Brighton, Carl T., Yoichi Sugioka, and Robert M. Hunt (1973), "Cytoplasmic structures of epiphyseal plate chondrocytes; quantitative evaluation using electron micrographs of rat costochondral junctions with specific reference to the fate of hypertrophic cells", Journal of Bone and Joint Surgery, 55-A: 771-784
- Brighton, Carl T. and Robert M. Hunt (1986): "Histochemical localization of calcium in the fracture callus with potassium pyroantimonate: possible role of chondrocyte mitochondrial calcium in callus calcification", Journal of Bone and Joint Surgery, 68-A (5): 703-715
- Sarem, Melika; Heizmann, Miriam; Barbero, Andrea; Martin, Ivan; Shastri, V. Prasad (2018-07-03). "Hyperstimulation of CaSR in human MSCs by biomimetic apatite inhibits endochondral ossification via temporal down-regulation of PTH1R". Proceedings of the National Academy of Sciences. 115 (27): E6135–E6144. Bibcode:2018PNAS..115E6135S. doi:10.1073/pnas.1805159115. ISSN 0027-8424. PMC 6142224. PMID 29915064.
- Ihde, Lauren L.; Forrester, Deborah M.; Gottsegen, Christopher J.; Masih, Sulabha; Patel, Dakshesh B.; Vachon, Linda A.; White, Eric A.; Matcuk, George R. (November 2011). "Sclerosing Bone Dysplasias: Review and Differentiation from Other Causes of Osteosclerosis". RadioGraphics. 31 (7): 1865–1882. doi:10.1148/rg.317115093. ISSN 0271-5333. PMID 22084176.