Epolactaene
Epolactaene is a neuritogenic fungal isolate.[1][2][3]
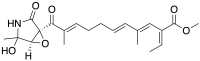 | |
| Names | |
|---|---|
| IUPAC name
Methyl (2E,3E,5E,9E)-2-ethylidene-11-[(1R,5R)-4-hydroxy-4-methyl-2-oxo-6-oxa-3-azabicyclo[3.1.0]hexan-1-yl]-4,10-dimethyl-11-oxoundeca-3,5,9-trienoate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C21H27NO6 | |
| Molar mass | 389.448 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Kakeya, H; Takahashi, I; Okada, G; Isono, K; Osada, H (1995). "Epolactaene, a novel neuritogenic compound in human neuroblastoma cells, produced by a marine fungus". The Journal of Antibiotics. 48 (7): 733–5. doi:10.7164/antibiotics.48.733. PMID 7649877.
- Nagumo Y, Kakeya H, Shoji M, Hayashi Y, Dohmae N, Osada H (2005). "Epolactaene binds human Hsp60 Cys442 resulting in the inhibition of chaperone activity". Biochem J. 387 (Pt 3): 835–40. doi:10.1042/BJ20041355. PMC 1135015. PMID 15603555.
- Mizushina Y, Kuramochi K, Ikawa H, Kuriyama I, Shimazaki N, Takemura M, et al. (2005). "Structural analysis of epolactaene derivatives as DNA polymerase inhibitors and anti-inflammatory compounds". Int J Mol Med. 15 (5): 785–93. doi:10.3892/ijmm.15.5.785. PMID 15806299.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.