Estrogen receptor test
The estrogen receptor test (ERT) is a laboratory test to determine whether cancer cells have estrogen receptors. This information can help establish how the cancer should be treated.[1]
The test uses immunohistochemical techniques on the estrogen receptor (ER) tumor marker. Immunohistochemistry (IHC) methods involve the selective identification of antigen proteins by exploiting antigen-antibody relationships.
Previously, the ligand binding assay was used to determine ER activity. However, this method was limited because of the large quantities of fresh tissue needed for each assay. IHC serves as a more efficient method as this technique allows for the tissue morphology to be observed in a tumor-specific manner. This increases the practicability of this technique as in many cases, patients’ tissue samples are limited in the applications of biomarker analysis. Anti-estrogen receptor antibodies were among the first of biomarkers that introduced a semi-quantitative assessment of ER activity.
Today, ER analysis is one of many routinely performed immunohistochemical assays performed to classify the hormone receptor status and to serve as a means of insight into the determination of cancer prognosis and management.
Estrogen receptor test
The ER activity can be monitored using immunohistochemical methods in applications like tissue analysis for tumorigenesis. The activation of the ER through binding to the estrogen hormone stimulates mammary cell division, catalyzing cell division and DNA replication processes. This results in exponential multiplication the mutated genes, along with large amounts of genotoxic waste, which in turn highly increase the chances of malignant cell formation and the likelihood of tumor formation.
Estrogen receptor types
There are two main types of estrogen receptors: estrogen receptor alpha (ERα) also known as NR3B1, and estrogen receptor beta (ER-β) also known as NR3A2. Both are nuclear receptors activated by the sex hormone estrogen and encoded by the ESR1 (Estrogen Receptor 1) gene. The estrogen signaling can be stimulated or inhibited selectively. This depends upon the equilibrium of these two receptor types in target organs.[2]
These two ER types are encoded by different genes located on separate chromosomes and have different functions. ERα is mostly active in the mammary gland and uterus, and aids in the regulation of skeletal homeostasis and metabolism.[3] ER-β on the other hand, plays a more prominent role in the central nervous and immune systems functional mechanisms.[3]
Immunohistochemical assessment
The ERT immunohistochemical assessment is a semi-quantitative method used to predict the likelihood of success to anti-estrogen therapy in breast carcinoma. ER-positive breast carcinomas are likely to respond to various endocrine treatments. Therefore, monitoring ER activity can be essential in disease and treatment progression.
Various target antibodies may be used in the IHC assessment of the ER. Typically, the antibody used for this experiment is the anti-Estrogen Receptor (ER) (SP1) Rabbit Monoclonal Antibody. Employing SP1 allows for detecting estrogen receptor (ER) antigens in sections of the fixed patient samples. In junction with light microscopy, the approximate ER activity can be estimated using the level of staining of the cell's components. The anti-ER (SP1) antibody targets the ER alpha protein (ERα) located in the nucleus of ER-positive normal and neoplastic cells.[4] The anti-ER (SP1) antibody's response is a useful indication of the progression, management, and prediction of therapy outcome of breast cancer. These antibodies are commercially available from 3 commonly used auto strain vendors- Dako, Leica, and Ventana. In a study by Kornaga et al, all behaved similarly in the semi-quantitative analysis of breast cancer biopsy samples.[4]
In a study in 2002, 6 breast carcinoma cases were received, characterized, and analyzed through the ERT IHC assessment. The level of known ER activity was classified (negative, low, medium, and high) and selected for observation. After embedment in a paraffin block, the samples were stained using a hematoxylin and eosin staining (H & E staining) system. The IHC analysis was then performed the same day using anti-ER monoclonal antibodies, and resulted in a consistently strong correlation in the carcinoma samples with ER activity. In contrast, the level of ER activity increased, while the IHC response increased respectively.[5]
Estrogen receptor test and breast cancer
A major challenge in cancer diagnosis and treatments is that the symptoms of many cancers are easily ignored or incorrectly credited to other ailments. The monitoring of irregularities or changes in receptor functions and be particularly useful in such cases.
Predictive biomarker assays seem to be the current gold standard for therapeutic interventions as they allow quantitative insights into the molecular environment. The estrogen receptor (ER) is no exception, as the observation of this receptor’s activity allows for insight into growth and proliferation and allows for differentiations to be made between various intercellular environments. The complex biochemical reactions of estrogen receptors are necessary for the mediation of cellular interactions in response to various cell-altering factors, including ligands, cofactors, and other simulative complexes.[6]
Monitoring tumor development with the estrogen receptor test

The estrogen receptor is a regulator of cellular functions, including cell growth and proliferation, and can serve as a means of inter-cellular differentiation.[6] Monitoring the activity of the ER via the ERT is necessary as it plays an essential role in normal breast development and function, as well as in cancerous situations. Accurate measurements of the ER activities are critical in the care and progression monitoring of breast cancers as the ER can serve as an indicative biomarker as it is a potential predictor for the clinical responses of a patient to cancer treatments. It has been proven that breast cancer patients who present an ER-positive status are most likely to respond to cancer treatments through endocrine therapy.[7] Measuring the ER activity is essential in the care of breast patients as the Estrogen receptor test allows for informed decisions to be made for the future best treatments for cancer patients.
Estrogen receptor test in mammary epithelial cancer
The ER activity is over-expressed in approximately 70% of diagnosed cases of breast cancers. Growing exposure of the mammary epithelium to estrogen is related to the risk of breast cancer as the binding of estrogen to the HER2 (cancerous breast cell receptor) in mammary cells causes a rise in the division and cell synthesis. This ultimately leads to a higher risk of replication errors, and the disruption of the normal cellular processes results in mistakes in apoptosis, cellular proliferation, or DNA repair.[8]
The ERT has been suggested as a predictor for the level of success of the use of endocrine therapy in cancer treatment. Many of the endocrine therapies for breast cancer treatments involve the use of selective estrogen receptor modulators (SERMs). SERMs for example, the drug tamoxifen, are ER antagonists in breast tissue. They are used in determining the sensitivity of breast cancer lesions to tamoxifen. Patients with ER-positive tumors are likely to respond well to these endocrine therapies.
Estrogen receptor pathway schematic
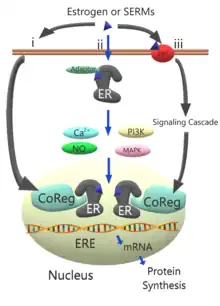
The schematic models the activity pathway of the estrogen receptor.
In the typical estrogen pathway (i) estrogen or other selective estrogen receptor modulators (SERMs) are bound to the estrogen receptor (ER) via the coregulatory proteins (CoReg) in the nucleus.[8]
Alternately, the estrogen or SERM complexes may occur through the ER located adjacent to the plasma membrane (ii) with the help of adaptor proteins such as caveolin 1 or SHC1, which target the ER complexes to the plasma membrane.[8] Activation of the ER complex causes increased kinase activity in phosphoinositide 3-kinases (PI3K) and mitogen-activated protein kinase (MAPK) as well as increases in concentrations of signaling molecules like calcium and nitric oxide.[8]
References
- "NCI Dictionary of Cancer Terms: estrogen receptor test". National Cancer Institute. National Institutes of Health. Retrieved 25 August 2023.
- Lee, Hye-Rim; Kim, Tae-Hee; Choi, Kyung-Chul (2012). "Functions and physiological roles of two types of estrogen receptors, ERα and ERβ, identified by estrogen receptor knockout mouse". Laboratory Animal Research. 28 (2): 71–6. doi:10.5625/lar.2012.28.2.71. PMC 3389841. PMID 22787479.
- Paterni, Ilaria; Granchi, Carlotta; Katzenellenbogen, John A.; Minutolo, Filippo (November 2014). "Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential". Steroids. 90: 13–29. doi:10.1016/j.steroids.2014.06.012. PMC 4192010. PMID 24971815.
- Kornaga EN, Klimowicz AC, Guggisberg N, Ogilvie T, Morris DG, Webster M, Magliocco AM (August 2016). "A systematic comparison of three commercial estrogen receptor assays in a single clinical outcome breast cancer cohort". Mod. Pathol. 29 (8): 799–809. doi:10.1038/modpathol.2016.74. PMID 27125355.
- von Wasielewski R, Mengel M, Wiese B, Rüdiger T, Müller-Hermelink HK, Kreipe H (November 2002). "Tissue array technology for testing interlaboratory and interobserver reproducibility of immunohistochemical estrogen receptor analysis in a large multicenter trial". Am. J. Clin. Pathol. 118 (5): 675–82. doi:10.1309/URLK-6AVK-331U-0V5P. PMID 12428786.
- Diaz LK, Sneige N (January 2005). "Estrogen receptor analysis for breast cancer: current issues and keys to increasing testing accuracy". Adv Anat Pathol. 12 (1): 10–9. doi:10.1097/00125480-200501000-00003. PMID 15614160.
- Lledge, R. M. E.; Reen, S. G.; Ugh, R. P.; Llred, D. C. A.; Lark, G. M. C.; Ill, J. H.; Avdin, P. R.; Artino, S. M.; Sborne, C. K. O. (2000). "Estrogen Receptor ( Er ) And Progesterone Receptor ( Pgr ), By Ligand-Binding Assay Compared With Er, Pgr And Ps2, By Immuno-Histochemistry In Predicting Response To Tamoxifen In Metastatic Breast Cancer : A Southwest Oncology Group Study". Int. J. Cancer (Pred. Oncol.). 89: 111–117. doi:10.1002/(sici)1097-0215(20000320)89:2<111::aid-ijc2>3.0.co;2-w.
- Deroo, B. J.; Korach, K. S. (1 March 2006). "Estrogen receptors and human disease". Journal of Clinical Investigation. 116 (3): 561–570. doi:10.1172/JCI27987. PMC 2373424. PMID 16511588.