Ethanesulfonic acid
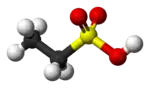
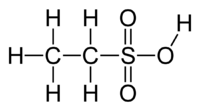
Ethanesulfonic acid (esylic acid) is a sulfonic acid with the chemical formula CH3CH2SO3H. The conjugate base is known as ethanesulfonate or, when used in pharmaceutical formulations, as esilate. It is a colorless liquid.[2]
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Ethanesulfonic acid | |||
| Other names
Esylic acid, ethylsulfonic acid | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.008.950 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H6O3S | |||
| Molar mass | 110.13 g/mol | ||
| Appearance | Colorless liquid | ||
| Density | 1.35 g/mL | ||
| Melting point | −17 °C (1 °F; 256 K) | ||
| Boiling point | 122–123 °C (252–253 °F; 395–396 K) at 0.01 mmHg | ||
| Soluble | |||
| log P | −0.37 | ||
| Acidity (pKa) | −1.68[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
References
- YK Ye & RW Stringham (2001-06-21). "Effect of mobile phase acidic additives on enantioselectivity for phenylalanine analogs". J Chromatogr A. 927 (1–2): 53–60. doi:10.1016/S0021-9673(01)01059-7. PMID 11572398.
- Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2000. doi:10.1002/14356007.a25_503.
External links
- Ye YK, Stringham RW (2006), The effect of acidic and basic additives on the enantioseparation of basic drugs using polysaccharide-based chiral stationary phases. Chirality 18, 519–530. (PubMed:16676332)
- National Center for Biotechnology Information (2005), Ethanesulfonic acid, PubChem Compound Database; CID=11668, (accessed December 23, 2016)
- https://www.scbt.com/scbt/product/ethanesulfonic-acid-594-45-6
- http://www.chemicalbook.com/productmsdsdetailcb5173859_en.htm
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.