Ferrate(VI)
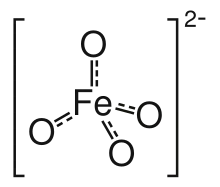
Ferrate(VI) is the inorganic anion with the chemical formula [FeO4]2−. It is photosensitive, contributes a pale violet colour to compounds and solutions containing it and is one of the strongest water-stable oxidizing species known. Although it is classified as a weak base, concentrated solutions containing ferrate(VI) are corrosive and attack the skin and are only stable at high pH. It is similar to the somewhat more stable permanganate.
 | |
 Solutions of ferrate (left) and permanganate (right) | |
| Names | |
|---|---|
| IUPAC name
Ferrate(VI) | |
| Systematic IUPAC name
Tetraoxoironbis(olate) | |
| Other names
[FeO4]2- | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| 2055 | |
PubChem CID |
|
| |
| |
| Properties | |
| [FeO4]2- | |
| Molar mass | 119.843 g mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Nomenclature
The term ferrate is normally used to mean ferrate(VI), although it can refer to other iron-containing anions, many of which are more commonly encountered than salts of [FeO4]2−. These include the highly reduced species disodium tetracarbonylferrate Na2[Fe(CO)4], K2[Fe(CO)4] and salts of the iron(III) complex tetrachloroferrate [FeCl4]− in 1-Butyl-3-methylimidazolium tetrachloroferrate. Although rarely studied, ferrate(V) [FeO4]3− and ferrate(IV) [FeO4]4− oxyanions of iron also exist. These too are called ferrates.[1]
Synthesis
Ferrate(VI) salts are formed by oxidizing iron in an aqueous medium with strong oxidizing agents under alkaline conditions, or in the solid state by heating a mixture of iron filings and powdered potassium nitrate.[2]
For example, ferrates are produced by heating iron(III) hydroxide with sodium hypochlorite in alkaline solution:[3]
The anion is typically precipitated as the barium(II) salt, forming barium ferrate.[3]
Properties
Fe(VI) is a strong oxidizing agent over the entire pH range, with a reduction potential (Fe(VI)/Fe(III) couple) varying from +2.2 V to +0.7 V versus SHE in acidic and basic media respectively.
- [FeO
4]2−
+ 8 H+ + 3 e− ⇌ Fe3+
+ 4 H2O; E0 = +2.20 V (acidic medium) - [FeO
4]2−
+ 4 H2O + 3 e− ⇌ Fe(OH)
3 + 5 OH−
; E0 = +0.72 V (basic medium)
Because of this, the ferrate(VI) anion is unstable at neutral[2] or acidic pH values, decomposing to iron(III):[3] The reduction goes through intermediate species in which iron has oxidation states +5 and +4.[4] These anions are even more reactive than ferrate(VI).[5] In alkaline conditions ferrates are more stable, lasting for about 8 to 9 hours at pH 8 or 9.[5]
Aqueous solutions of ferrates are pink when dilute, and deep red or purple at higher concentrations.[4][6] The ferrate ion is a stronger oxidizing agent than permanganate,[7] and oxidizes ammonia to molecular nitrogen.[8]
The ferrate(VI) ion has two unpaired electrons and is thus paramagnetic. It has a tetrahedral molecular geometry, isostructural with the chromate and permanganate ions.[4]
Applications
Ferrates are excellent disinfectants, and are capable of removing and destroying viruses.[9] They are also of interest as potential as an environmentally friendly water treatment chemical, as the byproduct of ferrate oxidation is the relatively benign iron(III).[10]
Sodium ferrate (Na2FeO4) is a useful reagent with good selectivity and is stable in aqueous solution of high pH, remaining soluble in an aqueous solution saturated with sodium hydroxide.
References
- Graham Hill; John Holman (2000). Chemistry in context (5th ed.). Nelson Thornes. p. 202. ISBN 0-17-448276-0.
- R. K. Sharma (2007). Text Book Of Coordination Chemistry. Discovery Publishing House. pp. 124–125. ISBN 978-81-8356-223-2.
- Gary Wulfsberg (1991). Principles of descriptive inorganic chemistry. University Science Books. pp. 142–143. ISBN 0-935702-66-0.
- Egon Wiberg; Nils Wiberg; Arnold Frederick Holleman (2001). Inorganic chemistry. Academic Press. pp. 1457–1458. ISBN 0-12-352651-5.
- Gary M. Brittenham (1994). Raymond J. Bergeron (ed.). The Development of Iron Chelators for Clinical Use. CRC Press. pp. 37–38. ISBN 0-8493-8679-9.
- John Daintith, ed. (2004). Oxford dictionary of chemistry (5th ed.). Oxford University Press. p. 235. ISBN 0-19-860918-3.
- Kenneth Malcolm Mackay; Rosemary Ann Mackay; W. Henderson (2002). Introduction to modern inorganic chemistry (6th ed.). CRC Press. pp. 334–335. ISBN 0-7487-6420-8.
- Karlis Svanks (June 1976). "Oxidation of Ammonia in Water by Ferrates(VI) and (IV)" (PDF). Water Resources Center, Ohio State University. p. 3. Retrieved 2010-05-04.
- Stanley E. Manahan (2005). Environmental chemistry (8th ed.). CRC Press. p. 234. ISBN 1-56670-633-5.
- Sharma, Virender K.; Zboril, Radek; Varma, Rajender S. (2015). "Ferrates: Greener Oxidants with Multimodal Action in Water Treatment Technologies". Accounts of Chemical Research. 48 (2): 182–191. doi:10.1021/ar5004219. ISSN 0001-4842. PMID 25668700.