Ferroquine
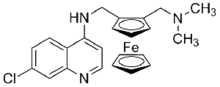
Ferroquine is a synthetic compound related to chloroquine which acts as an antimalarial, and shows good activity against chloroquine-resistant strains.[1] It contains an organometallic ferrocene ring which is unusual in pharmaceuticals,[2][3][4] and while it was first reported in 1997,[5] it has progressed slowly through clinical trials, with results from Phase II trials showing reasonable safety and efficacy, and further trials ongoing.[6][7]
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.208.008 |
| Chemical and physical data | |
| Formula | C23H24ClFeN3 |
| Molar mass | 433.76 g·mol−1 |
| |
References
- Domarle O, Blampain G, Agnaniet H, Nzadiyabi T, Lebibi J, Brocard J, et al. (March 1998). "In vitro antimalarial activity of a new organometallic analog, ferrocene-chloroquine". Antimicrobial Agents and Chemotherapy. 42 (3): 540–544. doi:10.1128/AAC.42.3.540. PMC 105495. PMID 9517929.
- Dive D, Biot C (March 2008). "Ferrocene conjugates of chloroquine and other antimalarials: the development of ferroquine, a new antimalarial". ChemMedChem. 3 (3): 383–391. doi:10.1002/cmdc.200700127. PMC 7162372. PMID 17806092.
- Biot C, Nosten F, Fraisse L, Ter-Minassian D, Khalife J, Dive D (August 2011). "The antimalarial ferroquine: from bench to clinic". Parasite. 18 (3): 207–214. doi:10.1051/parasite/2011183207. PMC 3671469. PMID 21894260.
- Wani WA, Jameel E, Baig U, Mumtazuddin S, Hun LT (August 2015). "Ferroquine and its derivatives: new generation of antimalarial agents". European Journal of Medicinal Chemistry. 101: 534–551. doi:10.1016/j.ejmech.2015.07.009. PMC 7115395. PMID 26188909.
- Biot C, Glorian G, Maciejewski LA, Brocard JS (November 1997). "Synthesis and antimalarial activity in vitro and in vivo of a new ferrocene-chloroquine analogue". Journal of Medicinal Chemistry. 40 (23): 3715–3718. doi:10.1021/jm970401y. PMID 9371235.
- Held J, Supan C, Salazar CL, Tinto H, Bonkian LN, Nahum A, et al. (December 2015). "Ferroquine and artesunate in African adults and children with Plasmodium falciparum malaria: a phase 2, multicentre, randomised, double-blind, dose-ranging, non-inferiority study". The Lancet. Infectious Diseases. 15 (12): 1409–1419. doi:10.1016/S1473-3099(15)00079-1. PMID 26342427.
- Adoke Y, Zoleko-Manego R, Ouoba S, Tiono AB, Kaguthi G, Bonzela JE, et al. (May 2021). "A randomized, double-blind, phase 2b study to investigate the efficacy, safety, tolerability and pharmacokinetics of a single-dose regimen of ferroquine with artefenomel in adults and children with uncomplicated Plasmodium falciparum malaria". Malaria Journal. 20 (1): 222. doi:10.1186/s12936-021-03749-4. PMC 8135182. PMID 34011358.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.