Fischer indole synthesis
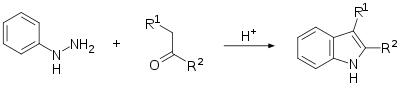
The Fischer indole synthesis is a chemical reaction that produces the aromatic heterocycle indole from a (substituted) phenylhydrazine and an aldehyde or ketone under acidic conditions.[1][2] The reaction was discovered in 1883 by Emil Fischer. Today antimigraine drugs of the triptan class are often synthesized by this method.
| Fischer indole synthesis | |
|---|---|
| Named after | Hermann Emil Fischer |
| Reaction type | Ring forming reaction |
| Identifiers | |
| Organic Chemistry Portal | fischer-indole-synthesis |
| RSC ontology ID | RXNO:0000064 |
This reaction can be catalyzed by Brønsted acids such as HCl, H2SO4, polyphosphoric acid and p-toluenesulfonic acid or Lewis acids such as boron trifluoride, zinc chloride, and aluminium chloride.
Reaction mechanism
The reaction of a (substituted) phenylhydrazine with a carbonyl (aldehyde or ketone) initially forms a phenylhydrazone which isomerizes to the respective enamine (or 'ene-hydrazine'). After protonation, a cyclic [3,3]-sigmatropic rearrangement occurs producing a diimine. The resulting diimine forms a cyclic aminoacetal (or aminal), which under acid catalysis eliminates NH3, resulting in the energetically favorable aromatic indole.
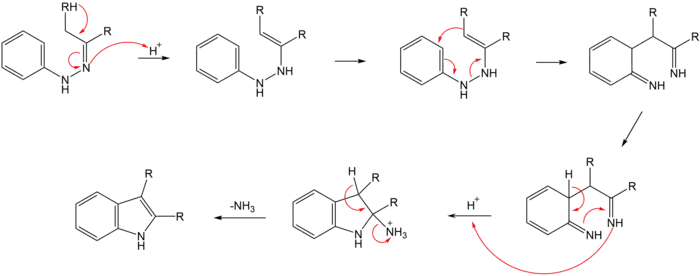
Isotopic labelling studies show that the aryl nitrogen (N1) of the starting phenylhydrazine is incorporated into the resulting indole.[6][7]
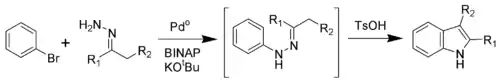
Buchwald modification
Via a palladium-catalyzed reaction, the Fischer indole synthesis can be effected by cross-coupling aryl bromides and hydrazones.[8] This result supports the previously proposed intermediacy as hydrazone intermediates in the classical Fischer indole synthesis. These N-arylhydrazones undergo exchange with other ketones, expanding the scope of this method.
Application
- Indometacin preparation.
- Triptan synthesis
- Iprindole synthesis (phenylhydrazine + suberone → 2,3-Cycloheptenoindole).
See also
- Bartoli indole synthesis
- Japp–Klingemann indole synthesis
- Leimgruber–Batcho indole synthesis
- Larock indole synthesis
Related reactions
References
- Fischer, E.; Jourdan, F. (1883). "Ueber die Hydrazine der Brenztraubensäure". Berichte der Deutschen Chemischen Gesellschaft. 16 (2): 2241–2245. doi:10.1002/cber.188301602141.
- Fischer, E.; Hess, O. (1884). "Synthese von Indolderivaten". Berichte der Deutschen Chemischen Gesellschaft. 17 (1): 559–568. doi:10.1002/cber.188401701155.
- van Order, R. B.; Lindwall, H. G. (1942). "Indole". Chemical Reviews. 30 (1): 69–96. doi:10.1021/cr60095a004.
- Robinson, B. (1963). "The Fischer Indole Synthesis". Chemical Reviews. 63 (4): 373–401. doi:10.1021/cr60224a003.
- Robinson, B. (1969). "Studies on the Fischer indole synthesis". Chemical Reviews. 69 (2): 227–250. doi:10.1021/cr60258a004.
- Allen, C. F. H.; Wilson, C. V. (1943). "The Use of N15 as a Tracer Element in Chemical Reactions. The Mechanism of the Fischer Indole Synthesis". Journal of the American Chemical Society. 65 (4): 611–612. doi:10.1021/ja01244a033.
- Clusius, K.; Weisser, H. R. (1952). "Reaktionen mit 15N. III. Zum Mechanismus der Fischer'schen Indolsynthese". Helvetica Chimica Acta. 35 (1): 400–406. doi:10.1002/hlca.19520350151.
- Wagaw, S.; Yang, B. H.; Buchwald, S. L. (1998). "A Palladium-Catalyzed Strategy for the Preparation of Indoles: A Novel Entry into the Fischer Indole Synthesis". Journal of the American Chemical Society. 120 (26): 6621–6622. doi:10.1021/ja981045r.