Fluoroethane
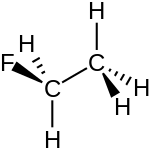
Fluoroethane (also known as ethyl fluoride) is a hydrofluorocarbon with the chemical formula C2H5F). It is a volatile derivative of ethane. It appears as a colourless, odorless flammable gas at room temperature.[3] Fluoroethane can also cause asphyxiation by the displacement of oxygen in air.[4]
 | |
| Names | |
|---|---|
| IUPAC name
Fluoroethane | |
| Other names
Ethyl fluoride, HFC-161 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.005.938 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 2453 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H5F | |
| Molar mass | 48.060 g·mol−1 |
| Appearance | Clear, colourless |
| Odor | Odorless |
| Hazards | |
| GHS labelling:[1] | |
  | |
| Danger | |
| H290, H314 | |
| P280, P305+P351+P338, P310 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LDLo (lowest published) |
26 pph/4H (rat, inhalation)[2] |
| Related compounds | |
Related compounds |
Fluoromethane; Fluoropropane; 1,1-Difluoroethane; 1,2-Difluoroethane;1,1,1-Trifluoroethane; 1,1,2-Trifluoroethane; Vinyl fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Reactivity
Fluoroethane is incompatible with most strong reducing agents and oxidizers. Also, may be incompatible with many amines, nitrides, azo/diazo compounds, with alkali metals, and with epoxides.[5] It is part of the wider class of substances known as fluorinated organic compounds.[6]
See also
References
- "System of Registries | US EPA". sor.epa.gov. Retrieved Sep 26, 2022.
- "Fluoroethane".
- PubChem. "Fluoroethane". pubchem.ncbi.nlm.nih.gov. Retrieved 2023-01-18.
- "ETHYL FLUORIDE | CAMEO Chemicals | NOAA". cameochemicals.noaa.gov. Retrieved 2023-01-18.
- PubChem. "Fluoroethane". pubchem.ncbi.nlm.nih.gov. Retrieved 2023-01-18.
- PubChem. "Fluoroethane". pubchem.ncbi.nlm.nih.gov. Retrieved 2023-01-18.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
