Forster–Decker method
The Forster–Decker method is a series of chemical reactions that transform a primary amine (1) ultimately to a secondary amine (6).[1][2] The first step is the formation of a Schiff base (3), followed by alkylation, and hydrolysis.
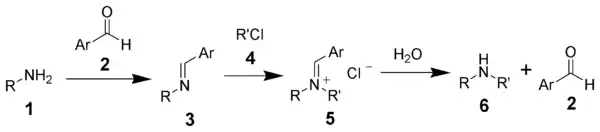
The Forster-Decker method
Mechanism
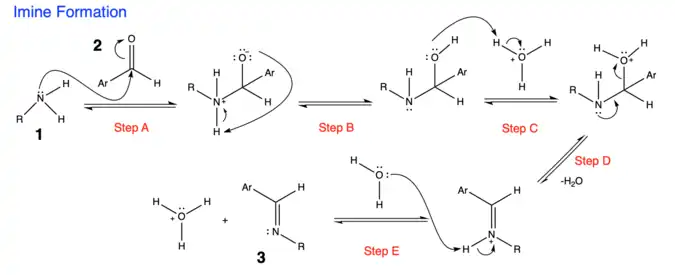
1. The first step in the Forster-Decker Method is the formation of an imine (Schiff base) from a primary amine. [3]
- Step A: Nucleophilic attack on the aldehyde by a primary amine
- Step B: Proton Transfer - creating carbinolamine
- Step C: Protonation of hydroxyl oxygen of carbinolamine
- Step D: Formation of iminium ion by losing water molecule
- Step E: Deprotonation giving imine product
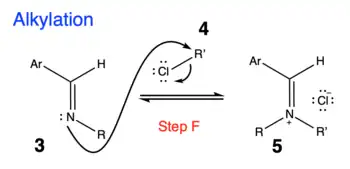
2. The next step is alkylation of the imine product to form iminium ion.[4]
- Step F: Alkylation of imine product with X-R' where X = Cl, Br or I
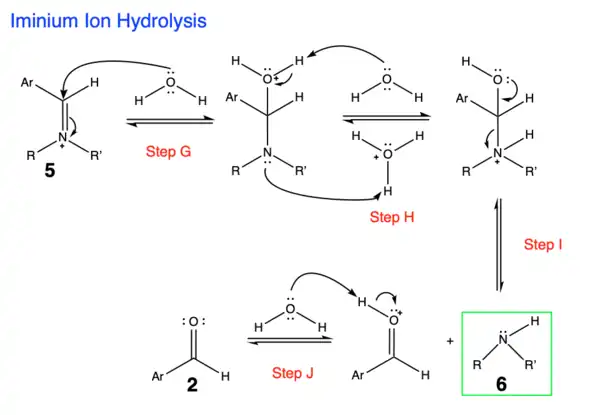
3. The final step is hydrolysis of the iminium ion to form a secondary amine and regenerate the aldehyde starting material. [5]
- Step G: Nucleophilic attack by a water molecule
- Step H: Proton transfers: shown in the mechanism using two molecules (water and acid) but this step might also be intramolecular
- Step I: Reformation of carbonyl and kicking out the secondary amine
- Step J: Deprotonation to regenerate aldehyde starting material
See also
References
- Forster, Martin Onslow (1899). "XCI.–Influence of substitution on specific rotation in the bornylamine series". Journal of the Chemical Society, Transactions. 75: 934–935. doi:10.1039/CT8997500934.
- Decker, H.; Becker, P. Ann. 1913, 395, 362.
- McMurry, John (1988). Organic Chemistry (Second ed.). Brooks/Cole Publishing Company. p. 674-675. ISBN 0-534-07968-7.
- "Forster-Decker Amine Synthesis". SynArchive.com The Organic Synthesis Archive. 2011–2022. Retrieved December 12, 2022.
- "Imine and Enamine Hydrolysis Mechanism". Chemistry Steps. 2016–2022. Retrieved December 13, 2022.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.