Freshwater phytoplankton
Freshwater phytoplankton is the phytoplankton occurring in freshwater ecosystems.[1] It can be distinguished between limnoplankton (lake phytoplankton), heleoplankton (phytoplankton in ponds), and potamoplankton (river phytoplankton).[2][3] They differ in size as the environment around them changes. They are affected negatively by the change in salinity in the water.[4]
Ecology
Temperature
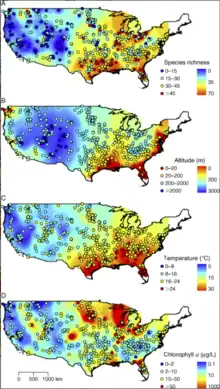
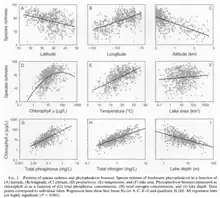
Temperature correlates with various characteristics of phytoplankton community: in a study of American freshwater plankton by Huisman et al,[5] higher temperatures correlates with increased species richness, increased overall biomass, and decreased cell size.[6] This may have serious implications under global warming, as given lower phytoplankton biomass with the same cell density would provide less food to the grazers and higher trophic levels, and smaller size may favour consumers that are able to access them at the expense of those that prefer larger algae.
Chlorophyll α
Chlorophyll α is the core photosynthetic pigment that all phytoplankton possess. Concentrations of this pigment, which can be measured remotely, is used as a proxy for phytoplankton biomass in a given location general, the more chlorophyll a, the more phytoplankton biomass, although the CHL a to C ratio May vary between species, and even within the species.
Water body characteristics
Freshwater phytoplankton shows a strong correlation with both surface area and depth of the water body they inhabit. Species richness increases in larger lakes with surface area and decreases in deeper lakes. Decreases due to depth are associated with lower chlorophyll α concentrations.
Salinity
Almost all freshwater phytoplankton die when salinity levels exceed 8%.[7] Between 0% and 8% however, some species may grow preferentially with some amount of salt available. This may be due to the presence of the salt itself, or the hydrodynamic processes that occur with water stratified due to unequal salinity.
Light Utilization
Cyanobacteria are adapted to low light environments and thus utilize light very efficiently. This is believed to be the result of the time period in which they evolved.[8] About 3.8 billion years ago solar luminosity was ~30% lower than present conditions. Cyanobacteria were able to adapt to this low light and thrive off the nutrient dense conditions.
Green algae are a high-light adapted group. They utilize light relatively inefficiently and need high levels of light to live.
Diatoms are competitive in low light, however, they do not use light as efficiently as cyanobacteria. They are adapted to mixed conditions that consist of interchanging periods of low and high light.
Mixotrophs, like dinoflagellates, do well in low light conditions. This may not be due to an efficient use of light however, but more so due to their ability to move independently and their mixotrophy.
Spring Bloom
The factor that promotes the occurring of spring bloom are light availability, phytoplankton physiology, nutrients, temperature, grazing, virus lysis. Phytoplankton begins to emerge when the sun begins to heat the surface of the water, creating a layered layer of warmer, less dense water that traps phytoplankton near the surface, where they are exposed to sunlight. Fresh water also has a positive effect on the growth of phytoplankton, since it is less dense, creates a layered water column and carries nutrients necessary for phytoplankton to carry out processes (photosynthesis). Collapse can be caused by nutrient depletion, vertical mixing when nutrients are at the bottom, resulting in less bloom. Also due to the high grazing pressure of zooplankton and decrease in illumination. [9][10]
Classification
A functional classification has been constructed to divide different species into different brackets based on: seasonal shifts, water body characteristics, morphology, nutrient availability, light intensity, as well as others. The current list contains 31 different alphanumeric terms that each represent a trait-differentiated functional group.[11]
References
- S., Reynolds, Colin (2006). Ecology of phytoplankton. Cambridge: Cambridge University Press. ISBN 978-0511191817. OCLC 76416312.
{{cite book}}: CS1 maint: multiple names: authors list (link) - S., Reynolds, Colin (1984). The ecology of freshwater phytoplankton. Cambridge: Cambridge University Press. ISBN 978-0521237826. OCLC 9442481.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Sandgren, Craig D. (1988). Growth and Reproductive Strategies of Freshwater Phytoplankton. Cambridge University Press. ISBN 9780521327220.
- Lionard, Marie; Muylaert, Koenraad; Gansbeke, Dirk Van; Vyverman, Wim (2005-05-01). "Influence of changes in salinity and light intensity on growth of phytoplankton communities from the Schelde river and estuary (Belgium/The Netherlands)". Hydrobiologia. 540 (1): 105–115. doi:10.1007/s10750-004-7123-x. ISSN 1573-5117. S2CID 39428857.
- Stomp, M., Huisman, J., Mittelbach, G.G., Litchman, E. and Klausmeier, C.A. (2011), Large‐scale biodiversity patterns in freshwater phytoplankton. Ecology, 92: 2096-2107. doi:10.1890/10-1023.1
- Zohary, Tamar & Flaim, Giovanna & Sommer, Ulrich. (2020). Temperature and the size of freshwater phytoplankton. Hydrobiologia. 10.1007/s10750-020-04246-6.
- Roselyn H. Jackson, P.J.le B. Williams, I.R. Joint, Freshwater phytoplankton in the low salinity region of the River Tamar estuary, Estuarine, Coastal and Shelf Science, Volume 25, Issue 3, 1987, Pages 299-311, ISSN 0272-7714, https://doi.org/10.1016/0272-7714(87)90073-4.
- Schwaderer, Anne S., Yoshiyama, Kohei, de Tezanos Pinto, Paula, Swenson, Nathan G., Klausmeier, Christopher A., Litchmana, Elena, (2011), Eco‐evolutionary differences in light utilization traits and distributions of freshwater phytoplankton, Limnology and Oceanography, 56, doi: 10.4319/lo.2011.56.2.0589.
- Zagarese, Horacio E.; Sagrario, María de los Ángeles González; Wolf-Gladrow, Dieter; Nõges, Peeter; Nõges, Tiina; Kangur, Külli; Matsuzaki, Shin-Ichiro S.; Kohzu, Ayato; Vanni, Michael J.; Özkundakci, Deniz; Echaniz, Santiago A.; Vignatti, Alicia; Grosman, Fabián; Sanzano, Pablo; Van Dam, Bryce; Knoll, Lesley B. (2021). "Patterns of CO2 concentration and inorganic carbon limitation of phytoplankton biomass in agriculturally eutrophic lakes". Water Research. 190: 116715. Bibcode:2021WatRe.19016715Z. doi:10.1016/j.watres.2020.116715. PMID 33310445. S2CID 229176393.
- Mabrouk, Lotfi; Hamza, Asma; Ben Mansour, Hedi (2021). "Factors controlling phytoplankton dynamics in an arid reservoir in Tunisia (Case of Sidi Saad dam)". Environmental Monitoring and Assessment. 193 (6): 354. doi:10.1007/s10661-021-09125-8. PMID 34028619. S2CID 235128085.
- Colin S. Reynolds, Vera Huszar, Carla Kruk, Luigi Naselli-Flores, Sergio Melo, Towards a functional classification of the freshwater phytoplankton, Journal of Plankton Research, Volume 24, Issue 5, May 2002, Pages 417–428, https://doi.org/10.1093/plankt/24.5.417