Humic substance
Humic substances (HS) are coloured recalcitrant organic compounds naturally formed during long-term decomposition and transformation of biomass residues. The colour of humic substances varies from yellow to brown to black. Humic substances represent the major part of organic matter in soil, peat, coal and sediments and are important components of dissolved natural organic matter (NOM) in lakes (especially, dystrophic lakes), rivers and sea water.


"Humic substances" is an umbrella term covering humic acid, fulvic acid, humin and hymatomelanic acid which differ in solubility. By definition, humic acid is soluble in water at neutral and alkaline pH, but insoluble at acidic pH < 2. Fulvic acid is soluble in water at any pH. Humin is not soluble in water at any pH. Hymatomelanic acid is part of humic acid that is soluble in ethanol.
This definition of humic substances is largely operational. It is rooted in the history of soil science and, more precisely, in the tradition of alkaline extraction, which dates back to 1786, when Franz Karl Achard treated peat with a solution of potassium hydroxide and, after subsequent addition of an acid, obtained an amorphous dark precipitate (i.e., humic acid). Aquatic humic substances were isolated for the first time later, in 1806, from spring water by Jöns Jakob Berzelius.
In terms of chemistry, fulvic acid, humic acid and humin share more similarities than differences and represent a continuum of humic molecules. All of them are constructed from similar aromatic, polyaromatic, aliphatic and carbohydrate units and contain the same functional groups (mainly, carboxylic, phenolic and ester) albeit in varying proportions.
Water solubility of humic substances is primarily governed by interplay of two factors: the amount of ionizable functional groups (mainly, carboxylic) and the molecular weight. In general, fulvic acid has higher amount of carboxylic groups and lower average molecular weight than humic acid. However, molecular weight distributions of humic and fulvic acids significantly overlap.
Age and origin of the source material determine the chemical structure of humic substances. In general, humic substances derived from soil and peat (which takes hundreds to thousands of years to form) have higher molecular weight, higher amount of functional groups, more carbohydrate units and less polyaromatic units than humic substances derived from leonardite (which takes millions of years to form).
Humic matter in isolation is the result of a chemical extraction from the soil organic matter or the dissolved organic matter and represent the humic molecules distributed in the soil or water.[1][2][3] A new understanding views humic substances not as high-molecular-weight macropolymers but as heterogeneous and relatively small molecular components of the soil organic matter auto-assembled in supramolecular associations and composed of a variety of compounds of biological origin and synthesized by abiotic and biotic reactions in soil.[4] It is the large molecular complexity of the soil humeome[5] that confers to humic matter its bioactivity in soil and its role as plant growth promoter.[6]
The academic definition of humic substances is under debate as "humification" becomes unsupported as a special case, leading to some radical definitions expanding HS to encompass all difficult-to-characterize soil organic matter, at the cost of clarity. There is also a call to forgo the traditional alkali extract method and directly analyze the soil, but its complexity prevents widespread adoption in agriculture.[7] In practice, this means some sources may apply a traditional acid-base analysis to compost, then state the results in term of "humic substances".[8]
Concepts of humic substances
The formation of humic substances in nature is one of the least understood aspects of humus chemistry and one of the most intriguing. There are three main theories to explain it: the lignin theory of Waksman (1932), the polyphenol theory, and the sugar-amine condensation theory of Maillard (1911).[9][10] Those theories are insufficient to account for observations in soil research.[7] Humic substances are formed by the microbial degradation of dead plant matter, such as lignin, cellulose and charcoal.[11][12] Humic substances in the lab are very resistant to further biodegradation. The structure, elemental composition and content of functional groups of a given sample depend on the water or soil source and the specific conditions of extraction. Nevertheless, the average properties of lab produced humic substances from different sources are remarkably similar.
Fractionation
Humic substances in soils and sediments can be divided into three main fractions: humic acids, fulvic acids, and humin. Their presence and relative abundance is inferred by lab extraction, a process which alters their original form beyond recognition.
- The humic and fulvic acids are extracted as a colloidal sol from soil and other solid phase sources into a strongly basic aqueous solution of sodium hydroxide or potassium hydroxide.[13]
- Humic acids are precipitated from this solution by adjusting the pH to 1 with hydrochloric acid.
- The alcohol-soluble portion of the humic fraction is, in general, named ulmic acid.
- So-called "gray humic acids" (GHA) are soluble in low-ionic-strength alkaline media.
- "Brown humic acids" (BHA) are soluble in alkaline conditions independent of ionic strength.
- The fulvic acids is left in solution at pH 1. They remain soluble independent of pH and ionic strength.[14]
- Humic acids are precipitated from this solution by adjusting the pH to 1 with hydrochloric acid.
- Humin are insoluble in dilute alkali.
Humic acid as traditionally produced in a laboratory is not a single acid; rather, it is a complex mixture of many different acids containing carboxyl and phenolate groups so that the mixture behaves functionally as a dibasic acid or, occasionally, as a tribasic acid. Humic acid used to amend soil is manufactured using these same well established procedures. Humic acids can form complexes with ions that are commonly found in the environment creating humic colloids.[15]
As a nutrition supplement, fulvic acid can be found in a liquid form as a component of mineral colloids. Fulvic acids are poly-electrolytes and are unique colloids that diffuse easily through membranes, whereas all other colloids do not.[16]
A sequential chemical fractionation called Humeomics can be used to isolate more homogeneous humic fractions and determine their molecular structures by advanced spectroscopic and chromatographic methods.[17] Substances identified in humic extracts and directly in soil include mono-, di-, and tri-hydroxycarboxylic acids, fatty acids, dicarboxylic acids, linear alcohols, phenolic acids, terpenoids, carbohydrates, and amino acids.[18]
Criticism
Decomposition products of dead plant materials form intimate associations with minerals, making it difficult to isolate and characterize soil organic constituents. 18th century soil chemists successfully used alkaline extraction to isolate a portion of the organic constituents in soil. This led to the theory that a 'humification' process created 'humic substances'; most commonly 'humic acid', 'fulvic acid', and 'humin'.[7] However, these humic substances have not been observed in soil.[19] Although 'humification' theory is unsupported by evidence, "the underlying theory persists in the contemporary literature, including current textbooks."[7] Attempts to redefine 'humic substances' in valid terms have resulted in a proliferation of incompatible definitions, "with far-reaching implications beyond our ability to communicate scientifically accurate soil processes and properties."[7]
Chemical characteristics
In nature
Since the dawn of modern chemistry, humic substances are among the most studied among the natural materials. Despite long study, their molecular structure remains elusive. The traditional view is that humic substances are heteropolycondensates, in varying associations with clay.[20] A more recent view is that relatively small molecules also play a role.[21] Humic substances account for 50 – 90% of cation exchange capacity. Similar to clay, char and colloidal humus hold cation nutrients.[22]
In traditional, base-soluble extracts
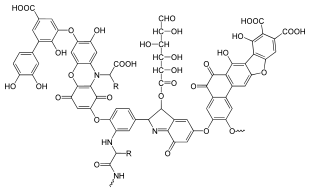
A typical humic substance is a mixture of many molecules, some of which are based on a motif of aromatic nuclei with phenolic and carboxylic substituents, linked together; the illustration shows a typical structure. The functional groups that contribute most to surface charge and reactivity of humic substances are phenolic and carboxylic groups.[23] Humic acids behave as mixtures of dibasic acids, with a pK1 value around 4 for protonation of carboxyl groups and around 8 for protonation of phenolate groups. There is considerable overall similarity among individual humic acids.[24] For this reason, measured pK values for a given sample are average values relating to the constituent species. The other important characteristic is charge density. The molecules may form a supramolecular structure held together by non-covalent forces, such as van der Waals force, π-π, and CH-π bonds.[21]
The presence of carboxylate and phenolate groups gives the humic acids the ability to form complexes with ions such as Mg2+, Ca2+, Fe2+, and Fe3+. Many humic acids have two or more of these groups arranged so as to enable the formation of chelate complexes.[25] The formation of (chelate) complexes is an important aspect of the biological role of humic acids in regulating bioavailability of metal ions.[24]
Determination of humic acids in water samples
The presence of humic acid in water intended for potable or industrial use can have a significant impact on the treatability of that water and the success of chemical disinfection processes. For instance, humic and fulvic acids can react with the chemicals used in the chlorination process to form disinfection byproducts such as dihaloacetonitriles, which are toxic to humans.[26][27] Accurate methods of establishing humic acid concentrations are therefore essential in maintaining water supplies, especially from upland peaty catchments in temperate climates.
As a lot of different bio-organic molecules in very diverse physical associations are mixed together in natural environments, it is cumbersome to measure their exact concentrations in the humic superstructure. For this reason, concentrations of humic acid are traditionally estimated out of concentrations of organic matter, typically from concentrations of total organic carbon (TOC) or dissolved organic carbon (DOC).
Extraction procedures are bound to alter some of the chemical linkages present in the soil humic substances (mainly ester bonds in biopolyesters such as cutins and suberins). The humic extracts are composed of large numbers of different bio-organic molecules that have not yet been totally separated and identified. However, single classes of residual biomolecules have been identified by selective extractions and chemical fractionation, and are represented by alkanoic and hydroxy alkanoic acids, resins, waxes, lignin residues, sugars, and peptides.
Ecological effects
Organic matter soil amendments have been known by farmers to be beneficial to plant growth for longer than recorded history.[28] However, the chemistry and function of the organic matter have been a subject of controversy since humans began postulating about it in the 18th century. Until the time of Liebig, it was supposed that humus was used directly by plants, but, after Liebig showed that plant growth depends upon inorganic compounds, many soil scientists held the view that organic matter was useful for fertility only as it was broken down with the release of its constituent nutrient elements into inorganic forms. At the present time, soil scientists hold a more holistic view and at least recognize that humus influences soil fertility through its effect on the water-holding capacity of the soil. Also, since plants have been shown to absorb and translocate the complex organic molecules of systemic insecticides, they can no longer discredit the idea that plants may be able to absorb the soluble forms of humus;[29] this may in fact be an essential process for the uptake of otherwise insoluble iron oxides.
A study on the effects of humic acid on plant growth was conducted at Ohio State University which said in part "humic acids increased plant growth" and that there were "relatively large responses at low application rates".[30]
A 1998 study by scientists at the North Carolina State University College of Agriculture and Life Sciences showed that addition of humate to soil significantly increased root mass in creeping bentgrass turf.[31][32]
A 2018 study by scientists at the University of Alberta showed that humic acids can reduce prion infectivity in laboratory experiments, but that this effect may be uncertain in the environment due to minerals in the soil that buffer the effect.[33]
Anthropogenic production
Humans can affect the production of humic substances via a variety of ways: by making use of natural processes by composting lignin or adding biochar (see soil rehabilitation), or by industrial synthesis of artificial humic substances from organic feedstocks directly. These artificial substances may be similarly divided into artificial humic acid (A-HA) and artificial fulvic acid (A-FA).[34]
Lignosulfonate from sulfite pulping can be made to mimic humus by hydrolysis and oxidation. The product is already commercialized as "lignohumate".[35]
Agricultural litter can be turned into an artificial humic substance by a hydrothermal reaction. The resulting mixture can increase the content of dissolved organic matter and total organic carbon in soil.[34]
Lignite (brown coal) may also be oxidized to produce humic substances, reversing the process of coal formation. This form of "mineral-derived fulvic acid" is widely used in China.[36] This process also occurs in nature, producing leonardite.[37]
Economic geology
In economic geology, the term humate refers to geological materials, such as weathered coal beds (leonardite), mudrock, or pore material in sandstones, that are rich in humic acids. Humate has been mined from the Fruitland Formation of New Mexico for use as a soil amendment since the 1970s, with nearly 60,000 metric tons produced by 2016.[38] Humate deposits may also play an important role in the genesis of uranium ore bodies.[39]
Technological applications
The heavy-metal binding abilities of humic acids have been exploited to develop remediation technologies for removing heavy metals from waste water. To this end, Yurishcheva et al. coated magnetic nanoparticles with humic acids. After capturing lead ions, the nanoparticles can then be captured using a magnet.[40]
Ancient masonry
Archeology finds that ancient Egypt used mudbricks reinforced with straw and humic acids.[41]
See also
References
- Piccolo A. (2016). "In memoriam of Prof. F.J. Stevenson and the question of humic substances". Chemical and Biological Technologies in Agriculture. 3. doi:10.1186/s40538-016-0076-2.
- Drosos M.; et al. (May 15, 2017). "A molecular zoom into soil Humeome by a direct sequential chemical fractionation of soi". The Science of the Total Environment. 586: 807–816. Bibcode:2017ScTEn.586..807D. doi:10.1016/j.scitotenv.2017.02.059. PMID 28214121.
- "Source Materials for International Humic Substances Society Samples". Retrieved 22 July 2020.
- Piccolo A.; et al. (2018). "The Molecular Composition of Humus Carbon: Recalcitrance and Reactivity in Soils". The Molecular Composition of Humus Carbon: Recalcitrance and Reactivity in Soils. In: The Future of Soil Carbon, Wiley and Sons. pp. 87–124. doi:10.1016/B978-0-12-811687-6.00004-3. ISBN 9780128116876.
- Nebbioso A. and Piccolo A. (2011). "Basis of a Humeomics Science: Chemical Fractionation and Molecular Characterization of Humic Biosuprastructures". Biomacromolecules. 12 (4): 1187–1199. doi:10.1021/bm101488e. PMID 21361272. S2CID 45333263.
- Canellas P.L and Olivares F.L. (2014). "TPhysiological responses to humic substances as plant growth promoter". Chemical and Biological Technologies in Agriculture. 1: 3. doi:10.1186/2196-5641-1-3.
- Lehmann, J.; Kleber, M. (2015-12-03). "The contentious nature of soil organic matter". Nature. 528 (7580): 60–8. Bibcode:2015Natur.528...60L. doi:10.1038/nature16069. PMID 26595271.
The development of this extraction method preceded theory, tempting scientists to develop explanations for the synthesis of materials resembling operationally extracted 'humic substances', rather than to develop an understanding of the nature of all organic matter in soil.[...] This lack of evidence means that 'humification' is increasingly questioned, yet the underlying theory persists in the contemporary literature, including current textbooks.[...] The issue has also been approached by redefining 'humic substances' as the portion of soil organic matter that cannot be molecularly characterized or by calling all soil organic matter 'humus'. We argue that this compromise - maintaining terminology but altering its meanings in varying ways — hampers scientific progress beyond the soil sciences. The [need for accurate models] of soil organic matter does not allow a confusing middle path; it requires leaving the traditional view behind to bring about lasting innovation and progress. This is critical as scientific fields outside the soil sciences base their research on the false premise of the existence of 'humic substances'. Thus an issue of terminology becomes a problem of false inference, with far-reaching implications beyond our ability to communicate scientifically accurate soil processes and properties.
- Palanivell, P; Susilawati, K; Ahmed, OH; Majid, NM (2013). "Compost and crude humic substances produced from selected wastes and their effects on Zea mays L. nutrient uptake and growth". The Scientific World Journal. 2013: 276235. doi:10.1155/2013/276235. PMC 3836416. PMID 24319353.
- Stevenson, F.J. (1994). Humus Chemistry: Genesis, Composition, Reactions, Wiley & Sons, New York, 1994, pp. 188-210. ISBN 0471594741.
- Tan, K. H. (2014). Humic matter in soil and the environment: principles and controversies. 2nd ed. Boca Ranton: CRC Press. ISBN 1482234459.
- Ponomarenko, E.V.; Anderson, D.W. (2001), "Importance of charred organic matter in Black Chernozem soils of Saskatchewan", Canadian Journal of Soil Science, 81 (3): 285–297, doi:10.4141/s00-075,
The present paradigm views humus as a system of heteropolycondensates, largely produced by the soil microflora, in varying associations with clay (Anderson 1979). Because this conceptual model, and simulation models rooted within the concept, do not accommodate a large char component, a considerable change in conceptual understanding (a paradigm shift) appears imminent.
- Mao, J.-D.; Johnson, R. L.; Lehmann, J.; Olk, D. C.; Neves, E. G.; Thompson, M. L.; Schmidt-Rohr, K. (2012). "Abundant and stable char residues in soils: implications for soil fertility and carbon sequestration". Environmental Science and Technology. 46 (17): 9571–9576. Bibcode:2012EnST...46.9571M. CiteSeerX 10.1.1.698.270. doi:10.1021/es301107c. PMID 22834642.
- Baigorri R; Fuentes M; González-Gaitano G; García-Mina JM; Almendros G; González-Vila FJ. (2009). "Complementary Multianalytical Approach To Study the Distinctive Structural Features of the Main Humic Fractions in Solution: Gray Humic Acid, Brown Humic Acid, and Fulvic Acid" (PDF). J Agric Food Chem. 57 (8): 3266–72. doi:10.1021/jf8035353. hdl:10261/58388. PMID 19281175.
- MacCarthy, Patrick (November 2001). "The Principles of Humic Substances". Soil Science. 166 (11): 738–751. Bibcode:2001SoilS.166..738M. doi:10.1097/00010694-200111000-00003. S2CID 101148229.
- "Effects of Humic Acid on Animals and Humans: An Overview of Literature and a Review of Current Research" (PDF). vet servis.
- Yamauchi, Masashige; Katayama, Sadamu; Todoroki, Toshiharu; Watanable, Toshio (1984). "Total synthesis of fulvic acid". Journal of the Chemical Society, Chemical Communications (23): 1565–6. doi:10.1039/C39840001565.
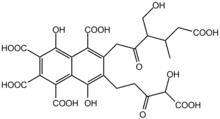
Synthesis of fulvic acid (1a) was accomplished by a route involving selective ozonization of 9-propenylpyranobenzopyran (1c), obtained by a regioselective cyclization of the 2-methylsulphinylmethyl 1,3-dione(3c)
(Note: this paper represents an attempt to produce fulvic acid, but the real extract is again a mixture of variable composition.) - Nebbioso A. and Piccolo A. (2012). "Advances in humeomics: Enhanced structural identification of humic molecules after size fractionation of a soil humic acid". Analytica Chimica Acta. 720: 77–90. doi:10.1016/j.aca.2012.01.027. PMID 22365124.
- Drosos M. and Piccolo A. (2018). "The molecular dynamics of soil humus as a function of tillage". Land Degradation & Development. 29 (6): 1792–1805. doi:10.1002/ldr.2989. S2CID 135445097.
- Weil, Ray R.; Brady, Nyle C. (2017). The Nature and Properties of Soils (15th ed.). Columbus, Ohio: Pearson Education (published April 2017). p. 549. ISBN 978-0-13-325448-8. LCCN 2016008568. OCLC 936004363.
[new analytical techniques have] found very little in the way of humic macromolecules in mineral soils. Instead, evidence suggests that the alkali extraction process itself actually creates giant polymers from smaller biomolecules.
- Ponomarenko, E.V.; Anderson, D.W. (2001), "Importance of charred organic matter in Black Chernozem soils of Saskatchewan", Canadian Journal of Soil Science, 81 (3): 285–297, doi:10.4141/s00-075
- Piccolo, A. (2002). The Supramolecular structure of humic substances. A novel understanding of humus chemistry and implications in soil science. Advances in Agronomy. Vol. 75. pp. 57–134. doi:10.1016/S0065-2113(02)75003-7. ISBN 978-0-12-000793-6.
- Weil, Ray R.; Brady, Nyle C. (2016). The Nature and Properties of Soils (15th ed.). Columbus: Pearson (published April 11, 2016). p. 554. ISBN 9780133254488. LCCN 2016008568. OCLC 942464649.
Humus accounts for 50 to 90% of cation exchange capacity. Like clays, humus colloids and high surface area char hold nutrient cations
- Stevenson F.J. (1994). Humus Chemistry: Genesis, Composition, Reactions. New York: John Wiley & Sons.
- Ghabbour, E.A.; Davies, G. (Editors) (2001). Humic Substances: Structures, Models and Functions. Cambridge, U.K.: RSC publishing. ISBN 978-0-85404-811-3.
{{cite book}}:|author2=has generic name (help) - Tipping, E (1994). "'WHAM – a chemical equilibrium model and computer code for waters, sediments, and soils incorporating a discrete site/electrostatic model of ion-binding by humic substances". Computers and Geosciences. 20 (6): 973–1023. Bibcode:1994CG.....20..973T. doi:10.1016/0098-3004(94)90038-8.
- Oliver, Barry G. (1983). "Dihaloacetonitriles in drinking water: Algae and fulvic acid as precursors". Environmental Science & Technology. 17 (2): 80–83. Bibcode:1983EnST...17...80O. doi:10.1021/es00108a003. PMID 22295957.
- Peters, Ruud J.B.; De Leer, Ed W.B.; De Galan, Leo (1990). "Dihaloacetonitriles in Dutch drinking waters". Water Research. 24 (6): 797. doi:10.1016/0043-1354(90)90038-8.
- Lapedes, Daniel N., ed. (1966). McGraw-Hill encyclopedia of science and technology: an international reference work, Volume 12. McGraw-Hill. p. 428. ISBN 978-0070452657.
The value of adding organic matter to the soil in the form of animal manures, green manures, and crop residues for producing favorable soil tilth has been known since ancient times
- Pan American Union. Dept. of Cultural Affairs. División de Fomento Científico, Pan American Union. Dept. of Scientific Affairs, Organization of American States. Dept. of Scientific Affairs (1984). Ciencia interamericana: Volumes 24–27.
And since plants have shown their ability to absorb and translocate the complex molecules of systemic insecticides, they can no longer discredit the idea that plants are able to absorb the soluble humic nutrients, containing by far ...
{{cite book}}: CS1 maint: multiple names: authors list (link) - Arancon, Norman Q.; Edwards, Clive. A.; Lee, Stephen; Byrne, Robert (2006). "Effects of humic acids from vermicomposts on plant growth". European Journal of Soil Biology. 42: S65–S69. CiteSeerX 10.1.1.486.2522. doi:10.1016/j.ejsobi.2006.06.004.
- Cooper, R. J.; Liu, Chunhua; Fisher, D. S. (1998). "Influence of Humic Substances on Rooting and Nutrient Content of Creeping Bentgrass". Crop Science. 38 (6): 1639. doi:10.2135/cropsci1998.0011183X003800060037x.
- Liu, Chunhua; Cooper, R. J. (August 1999). "Humic Substances Their Influence on Creeping Bentgrass Growth and Stress Tolerance" (PDF). TurfGrass Trends: 6.
- Kuznetsova, Alsu; Cullingham, Catherine; McKenzie, Debbie; Aiken, Judd M. (November 2018). "Soil humic acids degrade CWD prions and reduce infectivity". PLOS Pathogens. 14 (11): e1007414. doi:10.1371/journal.ppat.1007414. PMC 6264147. PMID 30496301.
- Tang, Chunyu; Li, Yuelei; Song, Jingpeng; Antonietti, Markus; Yang, Fan (2021-06-25). "Artificial humic substances improve microbial activity for binding CO2". iScience. 24 (6): 102647. Bibcode:2021iSci...24j2647T. doi:10.1016/j.isci.2021.102647. ISSN 2589-0042. PMC 8387571. PMID 34466779.
- Yakimenko, Olga; Stepanov, Andrey; Patsaeva, Svetlana; Khundzhua, Daria; Osipova, Olesya; Gladkov, Oleg (3 July 2021). "Formation of Humic-Like Substances during the Technological Process of Lignohumate® Synthesis as a Function of Time". Separations. 8 (7): 96. doi:10.3390/separations8070096.
- Gong, Guanqun; Xu, Liangwei; Zhang, Yingjie; Liu, Weixin; Wang, Ming; Zhao, Yufeng; Yuan, Xin; Li, Yajun (3 November 2020). "Extraction of Fulvic Acid from Lignite and Characterization of Its Functional Groups". ACS Omega. 5 (43): 27953–27961. doi:10.1021/acsomega.0c03388. PMC 7643152. PMID 33163778.
- "Youngs, R.W. & Frost, C.M. 1963. Humic acids from leonardite – a soil conditioner and organic fertilizer. Ind. Eng. Chem., 55, 95–99" (PDF). Archived from the original (PDF) on 2010-06-01. Retrieved 2022-03-18.
- Newcomer, Robert W.; Nybo, John P.; Newcomer, Jacob K. (2020). "Humate in the upper Cretaceous Fruitland Formation in northwestern New Mexico" (PDF). New Mexico Geological Society Special Publication. 14: 41–46. Retrieved 26 October 2020.
- McLemore, Virginia T. (2020). "Uranium deposits in the Poison Canyon trend, Ambrosia Lake Subdistrict, Grants Uranium District, McKinley and Cibola Counties, New Mexico" (PDF). New Mexico Geological Society Special Publication. 14: 53–63. Retrieved 26 October 2020.
- Yurishcheva, A.A.; Kydralieva, K.A.; Zaripova, A.A.; Dzhardimalieva, G.I.; Pomogaylo, A.D.; Jorobekova, S.J. (2013). "Sorption of Pb2+ by magnetite coated with humic acids". J. Biol. Phys. Chem. 13 (2): 61–68. doi:10.4024/36FR12A.jbpc.13.02.
- Lucas, A.; Harris, J.R. (1998). Ancient Egyptian Materials and Industries. New York: Dover Publications. p. 62. ISBN 978-0-486-40446-2.