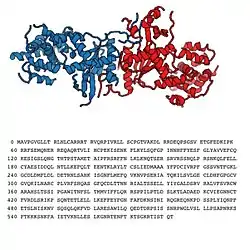
GLD-2
GLD-2 (which stands for Germ Line Development 2) is a cytoplasmic poly(A) polymerase (cytoPAPs) which adds successive AMP monomers to the 3’ end of specific RNAs, forming a poly(A) tail, which is a process known as polyadenylation.
For RNA specificity, GLD-2 associates with an RNA-binding protein, typically a GLD-3, to form a heterodimer that acts as a cytoplasmic PAP. This protein has an enzymatic function and belongs to a family (DNA polymerase type-B-like family) which includes several similar enzymes such as GLD-1, GLD-3 and GLD-4.
This family of cytoplasmic PAPs has been described in several different species including Homo sapiens, Caenorhabditis elegans, Xenopus, Mus musculus and Drosophila. Moreover, as it is a cytoplasmtaic PAP it differs from nuclear PAPs in some aspects. While nuclear PAPs contain a catalytic domain and an RNA-binding domain, GLD-2 family members have only a catalytic domain.
Localization
GLD-2 is a common and abundant, but yet quite unknown protein that has already been found in each of the five kingdoms. In the animal kingdom, it has been specially detected in Homo sapiens, Drosophila, Xenopus and Mus musculus. However, there has also been noticed the presence of GLD-2 in Arabidopsis thaliana which belongs in the plants kingdom; Escherichia Coli in monera and Candida albicans in fungi.
In human beings it is mostly expressed in the brain and within it, in the cerebellum, hippocampus and medulla. We can also find them in some other source tissues are the fibroblast, HeLa cell, MCF-7 cell, melanoma cell line and thymus. Inside those cells, it can be located in the nucleus and mitochondrion since its main function is related with DNA polyadenilation and these cell organelles are the only ones were DNA can be found. However, there are also GLD-2 in a soluble way in the cytosol, although the reason why they are there is still unsure.
In Escherichia Coli, this enzymatic protein can be found in the cell membrane and in the cytosol, whereas in Drosophila melanogaster, it predominates in the brain's nucleus and cytoplasm, oocyte, ovary and testis’ cells. Finally, in the Arabidopsis thaliana, it is located in the flower's nucleus, root, stem and leaf cells.
Related functions
GLD-2 primarily stabilizes mRNAs that are translationally repressed as well as it strongly promotes bulk polyadenylation. Surprisingly, those functions seem to have little impact on dynamizing efficient target mRNA translation, as it is an efficient Poly(A) Polymerase which helps developing polyadenylation activity. This activity is stimulated by its interaction with a putative RNA-binding protein: GLD-3. It is proposed by some studies that GLD-3 stimulates GLD-2 by recruiting it to the RNA. If so, then bringing GLD-2 to the RNA by other means also should stimulate its activity.
ATP binding
GLD-2, as a poly(A) polymerase (PAP) acts incorporating ATP at the 3' end of mRNAs in a template-independent manner.
Enzymatic activity: Polynucleotide adenylyltransferase activity
It has been discovered that this protein has a catalytic activity, in other words, it has the ability to increase the speed of chemical reactions which would not occur so fast. It is known to catalysis the following reaction (which requires the following cofactor: Mg(2+)):
ATP + RNA(n) ⇄ diphosphate + RNA(n+1)
Depending on the surroundings the optimal pH varies from 8 in the cytoplasm to 8.3 in the nucleus.
Hematopoietic progenitor cell differentiation
The GLD-2 protein together with 136 proteins more, is involved in the molecular process of hematopoietic progenitor cell differentiation, in the human proteome. This is the process in which precursor cell type acquires the specialized features of a hematopoietic progenitor cell, a kind of cell types including myeloid progenitor cells and lymphoid progenitor cells.
mRNA processing by RNA polyadenylation
The polyadenylation activity of GLD-2, as we previously mentioned, is stimulated by physical interaction with an RNA binding protein, GLD-3. To test whether GLD-3 might stimulate GLD-2 by recruiting it to RNA, some studies tethered C. elegans GLD-2 to mRNAs in Xenopus oocytes by using MS2 coat protein. Tethered GLD-2 adds poly(A) and stimulates translation of the mRNA, demonstrating that recruitment is sufficient to stimulate polyadenylation activity. PAP heterodimer in which GLD-2 contains the active site and GLD-3 provides RNA-binding specificity. MS2 coat protein was joined to GLD-2 to recruit it to an RNA.
Furthermore, GLD-2 activity is also important to maintain or up-regulate the abundance of many mRNAs, as the cytoplasmic polyadenylation has an essential role in activating maternal mRNA translation during early development. In vertebrates, the reaction requires CPEB, an RNA-binding protein and the poly(A) polymerase GLD-2.
The Xenopus enzyme, which exists in two closely related forms, polyadenylates RNAs to which it is tethered and enhances their translation. Likewise, it interacts with cytoplasmic polyadenylation factors, including Cleavage and polyadenylation specificity factor and CPEB, and with target mRNAs. These findings confirm and extend a recent report that a GLD-2 enzyme is the long-sought PAP responsible for cytoplasmic polyadenylation in oocytes.
In addition, the formation of long-term memory is believed to lack translational control of localized mRNAs. In mammals, dendrite mRNAs are kept in a repressed state and are activated upon repetitive stimulation. Several regulatory proteins required for translational control in early development are thought to be needed for memory formation, suggesting similar molecular mechanisms. In an experiment using Drosophila, it has been detected the enzyme responsible for poly(A) elongation in the brain and it has been demonstrated too that its activity is required specifically for long-term memory. These findings provide strong evidence that cytoplasmic polyadenylation is critical for memory formation, and that GLD2 is the responsible enzyme.
Medical implications
It has also been discovered that GLD2 has medical uses.
For example, such enzyme is overexpressed in patients who suffer from cancer; that's why it can be used as a prognostic factor for early appearance in breast cancer patients. Moreover, PAP activity is used to measure the effect of anticancer drugs as etoposide and cordycepin in two carcinoma cell lines: HeLa, which is the human epithelioid cervix carcinoma, and MCF-7 (human breast cancer). However, in spite its utilities it can also be involved in the expression of several common diseases such as: leukemia, liver cirrhosis, brain injuries, hepatitis and in some cases infertility in male patients.
References
- GRCh38: Ensembl release 89: ENSG00000164329 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
Further reading
- "UniProtKB - Q6PIY7 (GLD2_HUMAN)". UniProt.
- Nousch M, Yeroslaviz A, Habermann B, Eckmann CR (October 2014). "The cytoplasmic poly(A) polymerases GLD-2 and GLD-4 promote general gene expression via distinct mechanisms". Nucleic Acids Research. Oxford Journals. 42 (18): 11622–33. doi:10.1093/nar/gku838. PMC 4191412. PMID 25217583.
- "GLD-2". InteractiveFly: GeneBrief.
- Kwak JE, Wang L, Ballantyne S, Kimble J, Wickens M (March 2004). "Mammalian GLD-2 homologs are poly(A) polymerases". Proceedings of the National Academy of Sciences of the United States of America. 101 (13): 4407–12. Bibcode:2004PNAS..101.4407K. doi:10.1073/pnas.0400779101. PMC 384760. PMID 15070731.
- Kwak JE, Wickens M (June 2007). "A family of poly(U) polymerases". RNA. 13 (6): 860–7. doi:10.1261/rna.514007. PMC 1869031. PMID 17449726.
- Martin G, Möglich A, Keller W, Doublié S (August 2004). "Biochemical and structural insights into substrate binding and catalytic mechanism of mammalian poly(A) polymerase". Journal of Molecular Biology. 341 (4): 911–25. doi:10.1016/j.jmb.2004.06.047. PMID 15328606.
- "PAPD4 » Poly(A) RNA polymerase GLD2 [ EC 2.7.7.19 ]". Nexprot BETA.
- "GO:0002244 » Hematopoietic progenitor cell differentiation". NextProt BETA.
- Nousch M, Yeroslaviz A, Habermann B, Eckmann CR (October 2014). "The cytoplasmic poly(A) polymerases GLD-2 and GLD-4 promote general gene expression via distinct mechanisms". Nucleic Acids Research. 42 (18): 11622–33. doi:10.1093/nar/gku838. PMC 4191412. PMID 25217583.
- Nakel K, Bonneau F, Eckmann CR, Conti E (July 2015). "Structural basis for the activation of the C. elegans noncanonical cytoplasmic poly(A)-polymerase GLD-2 by GLD-3". Proceedings of the National Academy of Sciences of the United States of America. 112 (28): 8614–9. Bibcode:2015PNAS..112.8614N. doi:10.1073/pnas.1504648112. PMC 4507228. PMID 26124149.
- "Information on EC 2.7.7.19 - polynucleotide adenylyltransferase". BRENDA Data Base.