Galactogen
Galactogen is a polysaccharide of galactose that functions as energy storage in pulmonate snails and some Caenogastropoda.[1] This polysaccharide is exclusive of the reproduction and is only found in the albumen gland from the female snail reproductive system and in the perivitelline fluid of eggs.
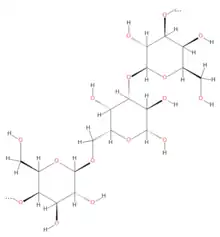 General structure of the polysaccharide galactogen | |
| Names | |
|---|---|
| IUPAC name
beta-D-galacto-hexopyranosyl-(1->3)-[beta-D-galacto-hexopyranosyl-(1->6)]-beta-D-galacto-hexopyranose | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI |
|
| ChemSpider |
|
| KEGG |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Galactogen serves as an energy reserve for developing embryos and hatchlings, which is later replaced by glycogen in juveniles and adults.[2] The advantage of accumulating galactogen instead of glycogen in eggs remains unclear,[3] although some hypotheses have been proposed (see below).
Occurrence and distribution
Galactogen has been reported in the albumen gland of pulmonate snails such as Helix pomatia,[4] Limnaea stagnalis,[5] Oxychilus cellarius,[6] Achatina fulica,[7] Aplexa nitens and Otala lactea,[8] Bulimnaea megasoma,[9] Ariolimax columbianis,[10] Ariophanta,[11] Biomphalaria glabrata,[12] and Strophochelius oblongus.[13] This polysaccharide was also identified in the Caenogastropoda Pila virens and Viviparus,[11] Pomacea canaliculata, [14] and Pomacea maculata.[15]
In adult gastropods, galactogen is confined to the albumen gland, showing a large variation in content during the year and reaching a higher peak in the reproductive season.[2] During the reproductive season, this polysaccharide is rapidly restored in the albumen gland after being transferred to the eggs, decreasing its total amount only after repeated ovipositions.[16][17] In Pomacea canaliculata snails, galactogen would act, together with perivitellins, as a main limiting factor of reproduction.[17] This polysaccharide has been identified in the Golgi zone of the secretory cells from the albumen gland in the form of discrete granules 200 Å in diameter.[18][19][20] The appearance of galactogen granules within the secretory globules suggests that this is the site of biosynthesis of the polysaccharide.[1][20]
Apart from the albumen gland, galactogen is also found as a major component of the perivitelline fluid from the snail eggs, comprising the main energy source for the developing embryo.[4][5][14][15]
Structure
Galactogen is a polymer of galactose with species-specific structural variations. In this polysaccharide, the D-galactose are predominantly β (1→3) and β (1→6) linked; however some species also have β (1→2) and β (1→4).[3] The galactogen of the aquatic Basommatophora (e.g. Lymnaea, Biomphalaria) is highly branched with only 5-8 % of the sugar residues in linear sections, and β(1→3) and β(1→6) bonds alternate more-or-Iess regularly. In the terrestrial Stylommatophora (e.g. Helix, Arianta, Cepaea, Achatina) up to 20% of the sugar residues are linear β(1→3) bound. The galactogen of Ampullarius sp species has an unusually large proportion of linearly arranged sugars, with 5% β(1→3), 26% β(1→6), and 10% β(1→2).[3] Other analyses in Helix pomatia suggested a dichotomous structure, where each galactopyranose unit bears a branch or side chain.[21][22]
Molecular weight determinations in galactogen extracted from the eggs of Helix pomatia and Limnaea stagnalis were estimated in 4x106 and 2.2x106, respectively.[23][24] In these snails galactogen contains only D-galactose.[25] Depending upon the origin of the galactogen, apart from D-galactose, L-galactose, L-fucose, D-glucose, L-glucose and phosphate residues may also be present;[3] for instance, the galactogen from Ampullarius sp. contains 98% of D-galacotose and 2% of L- fucose,[26] and the one isolated from Pomacea maculata eggs consist in 68% of D-galactose and 32% of D-glucose.[15] Phosphate-substituted galactose residues are found in the galactogen of individual species from various snail genera such as Biomphalaria, Helix and Cepaea.[27] Therefore, current knowledge indicates it could be considered either a homopolysaccharide of or a heteropolysaccharide dominated by galactose.
Metabolism
Galactogen is synthesized by secretory cells in the albumen gland of adult female snails and later transferred to the egg. This process is under neurohormonal control,[9][28] notably by the brain galactogenin.[29] The biochemical pathways for glycogen and galactogen synthesis are closely related. Both use glucose as a common precursor and its conversion to activated galactose is catalyzed by UDP-glucose 4-epimerase and galactose-1-P uridyl-transferase. This enables glucose to be the common precursor for both glycogenesis and galactogenesis.[30] In fact, both polysaccharides are found in the same secretory cells of the albumen gland and are subject to independent seasonal variations.[19] Glycogen accumulates in autumn as a general energy storage for hibernation, whereas galactogen is synthesized during spring in preparation of egg-laying.[31] It is commonly accepted that galactogen production is restricted to embryo nutrition and therefore is mainly transferred to eggs.
Little is known about the galactogen-synthesizing enzymes. A D-galactosyltransferase was described in the albumen gland of Helix pomatia.[32] This enzyme catalyzes the transfer of D-galactose to a (1→6) linkage and is dependent upon the presence of acceptor galactogen. Similarly, a β-(1→3)-galactosyltransferase activity has been detected in albumen gland extracts from Limnaea stagnalis.[33]
In embryos and fasting newly hatched snails, galactogen is most likely an important donor (via galactose) of metabolic intermediates. In feeding snails, the primary diet is glucose-containing starch and cellulose. These polymers are digested and contribute glucose to the pathways of intermediary metabolism.[1] Galactogen consumption begins at the gastrula stage and continues throughout development. Up to 46-78 % of egg galactogen disappears during embryo development. The remainder is used up within the first days after hatching.[9]
Only snail embryos and hatchlings are able to degrade galactogen, whereas other animals and even adult snails do not.[9][34][35] β-galactosidase may be important in the release of galactose from galactogen; however, most of the catabolic pathway of this polysaccharide is still unknown.[1]
Other functions
Besides being a source of energy, few other functions have been described for galactogen in the snail eggs, and all of them are related to embryo defense and protection. Given that carbohydrates retain water, the high amount of this polysaccharide would protect the eggs from desiccation from those snails that have aerial oviposition.[36][37] Besides, the high viscosity that the polysaccharide may confer to the perivitelline fluid has been suggested as a potential antimicrobial defense.[37]
Since galactogen is a β-linked polysaccharide, such as cellulose or hemicelluloses, specific biochemical adaptations are needed to exploit it as a nutrient, such as specific glycosidases. However, apart from snail embryos and hatchlings, no animal seems to be able to catabolize galactogen, including adult snails. This fact led to consider galactogen as part of an antipredation defense system exclusive of gastropods, deterring predators by lowering the nutritional value of eggs.[15]
References
- Goudsmit EM (1972). "Carbohydrates and carbohydrate metabolism in Mollusca.". In Florkin M, Scheer BT (eds.). Chemical Zoology. Vol. VII Mollusca. New York: Academic Press. pp. 219–244.
- May F (1932). "Beitrag zur Kenntnis des Glykogen und Galaktogengehaltes bei Helix pomatia". Z. Biol. 92: 319–324.
- Urich K (1994). Comparative Animal Biochemistry. Springer Berlin Heidelberg. pp. 1–8. doi:10.1007/978-3-662-06303-3_1. ISBN 978-3-642-08181-1.
- May F (1932). "Ober den Galactogengehalt der Eier von Heilix pomatia". Z. Biol. 92: 325–330.
- Horstmann HJ (1965). "Studies on the galactogen metabolism in the snail (Helix pomatia L.). 3. Catabolism of galactogen in young animals". Z. Biol. 115 (2): 133–155. PMID 5847702.
- Rigby JE (2009-08-20). "Alimentary and Reproductive Systems of Oxychilus Cellarius (Müller) (Stylommatophora)". Proceedings of the Zoological Society of London. 141 (2): 311–359. doi:10.1111/j.1469-7998.1963.tb01615.x.
- Ghose KC (1963). "Reproductive System of the Snail Achatina Fulica". Proceedings of the Zoological Society of London. 140 (4): 681–695. doi:10.1111/j.1469-7998.1963.tb01993.x. ISSN 1469-7998.
- McMAHON P, Von Brand T, Nolan MO (October 1957). "Observations on the polysaccharides of aquatic snails". Journal of Cellular and Comparative Physiology. 50 (2): 219–40. doi:10.1002/jcp.1030500206. PMID 13513668.
- Goudsmit EM (January 1976). "Galactogen catabolism by embryos of the freshwater snails, Bulimnaea megasoma and Lymnaea stagnalis". Comparative Biochemistry and Physiology. B, Comparative Biochemistry. 53 (4): 439–42. doi:10.1016/0305-0491(76)90194-2. PMID 4280.
- Meenakshi VR, Scheer BT (May 1969). "Regulation of galactogen synthesis in the slug Ariolimax columbianus". Comparative Biochemistry and Physiology. 29 (2): 841–845. doi:10.1016/0010-406x(69)91636-3. ISSN 0010-406X.
- Meenakshi VR (September 1954). "Galactogen in some common south Indian gastropods with special reference to Pila". Current Science. 23 (9): 301–2. JSTOR 24054957.
- Corrêa JB, Dmytraczenko A, Duarte JH (February 1967). "Structure of a galactan found in the albumen gland of Biomphalaria glabrata". Carbohydrate Research. 3 (4): 445–452. doi:10.1016/s0008-6215(00)81676-6. ISSN 0008-6215.
- Duarte JH, Jones JK (February 1971). "Some structural studies on the galactan from the albumen glands of the snail, Strophocheilus oblongus". Carbohydrate Research. 16 (2): 327–335. doi:10.1016/s0008-6215(00)81168-4. ISSN 0008-6215.
- Heras H, Garin CF, Pollero RJ (1998). "Biochemical composition and energy sources during embryo development and in early juveniles of the snail Pomacea canaliculata (Mollusca: Gastropoda)". Journal of Experimental Zoology. 280 (6): 375–383. doi:10.1002/(SICI)1097-010X(19980415)280:6<375::AID-JEZ1>3.0.CO;2-K. ISSN 1097-010X.
- Giglio ML, Ituarte S, Pasquevich MY, Heras H (2016-09-12). "The eggs of the apple snail Pomacea maculata are defended by indigestible polysaccharides and toxic proteins". Canadian Journal of Zoology. 94 (11): 777–785. doi:10.1139/cjz-2016-0049. hdl:1807/74381. ISSN 0008-4301.
- Wijsman TC, van Wijck-Batenburg H (1987-09-01). "Biochemical Composition of the Eggs of the Freshwater Snail Lymnaea stagnalis and Oviposition-induced Restoration of Albumen Gland Secretion". International Journal of Invertebrate Reproduction and Development. 12 (2): 199–212. doi:10.1080/01688170.1987.10510317. ISSN 0168-8170.
- Cadierno MP, Saveanu L, Dreon MS, Martín PR, Heras H (August 2018). "Biosynthesis in the Albumen Gland-Capsule Gland Complex Limits Reproductive Effort in the Invasive Apple Snail Pomacea canaliculata". The Biological Bulletin. 235 (1): 1–11. doi:10.1086/699200. PMID 30160995. S2CID 52135669.
- Grainger JN, Shilitoe AJ (March 1952). "Histochemical observations on galactogen". Stain Technology. 27 (2): 81–5. doi:10.3109/10520295209105064. PMID 14931082.
- Nieland ML, Goudsmit EM (October 1969). "Ultrastructure of galactogen in the albumen gland of Helix pomatia". Journal of Ultrastructure Research. 29 (1–2): 119–40. doi:10.1016/s0022-5320(69)80059-6. PMID 4310741.
- Catalán M, Dreon MS, Heras H, Pollero RJ, Fernández SN, Winik B (June 2006). "Pallial oviduct of Pomacea canaliculata (Gastropoda): ultrastructural studies of the parenchymal cellular types involved in the metabolism of perivitellins". Cell and Tissue Research. 324 (3): 523–33. doi:10.1007/s00441-005-0132-x. PMID 16453107. S2CID 30906846.
- O'Colla P (January 1952). "The application of the Barry degradation to snail galactogen". Proceedings of the Royal Irish Academy. Section B: Biological, Geological, and Chemical Science. Royal Irish Academy. 55: 165–170. JSTOR 20490896.
- Baldwin E, Bell DJ (1938-01-01). "278. A preliminary investigation of galactogen from the albumin glands of Helix pomatia". Journal of the Chemical Society (Resumed): 1461–1465. doi:10.1039/JR9380001461. ISSN 0368-1769. Retrieved 2020-08-17.
- Horstmann HJ (November 1964). "[Studies on Galactogen Metabolism of Snails (Helix Pomatia L.) I. The Preparation and Properties of Native Galactogen From Eggs]". Biochemische Zeitschrift. 340: 548–51. PMID 14331584.
- Fleitz H, Horstmann HJ (1967-01-01). "Über das native Galaktogen aus den Eiern der Schlammschnecke Lymnaea stagnalis". Biological Chemistry. 348 (Jahresband): 1301–1306. doi:10.1515/bchm2.1967.348.1.1301. ISSN 1437-4315. Retrieved 2020-08-17.
- Horstmann HJ, Geldmacher-Mallinckrodt M (September 1961). "[Research on metabolism in air-breathing snails. III. Galactogen in the eggs of Lymnaea stagnalis L]". Hoppe-Seyler's Zeitschrift für Physiologische Chemie. 325 (Jahresband): 251–9. doi:10.1515/bchm2.1961.325.1.251. PMID 14449055. Retrieved 2020-08-17.
- Feijó ML, Duarte JH (1975-11-01). "Some structural studies on the fucogalactan from egg masses of the snail ampullarius sp". Carbohydrate Research. 44 (2): 241–249. doi:10.1016/S0008-6215(00)84167-1. ISSN 0008-6215.
- Holst O, Mayer H, Okotore RO, König WA (1984-12-01). "Structural Studies on the Galactan from the Albumin Gland of Achatina fulica". Zeitschrift für Naturforschung C. 39 (11–12): 1063–1065. doi:10.1515/znc-1984-11-1211. ISSN 1865-7125. S2CID 6008556.
- Goudsmit EM (August 1978). "Calcium-dependent release of a neurochemical messenger from the brain of the land snail, Helix pomatia". Brain Research. 151 (2): 418–23. doi:10.1016/0006-8993(78)90900-9. PMID 28169. S2CID 37817654.
- Goudsmit EM, Ram JL (January 1982). "Stimulation of Helix pomatia albumen gland galactogen synthesis by putative neurohormone (galactogenin) and by cyclic AMP analogues". Comparative Biochemistry and Physiology Part B: Comparative Biochemistry. 71 (3): 417–422. doi:10.1016/0305-0491(82)90403-5. ISSN 0305-0491.
- Livingstone DR, De Zwaan A (1983). Metabolic Biochemistry and Molecular Biomechanics. Elsevier. pp. 177–242. doi:10.1016/b978-0-12-751401-7.50012-0. ISBN 978-0-12-751401-7.
- May FJ (1934). Chemische und biologische Untersuchungen über Galaktogen. Lehmann. OCLC 256810367.
- Goudsmit EM, Ketchum PA, Grossens MK, Blake DA (September 1989). "Biosynthesis of galactogen: identification of a beta-(1----6)-D-galactosyltransferase in Helix pomatia albumen glands". Biochimica et Biophysica Acta. 992 (3): 289–97. doi:10.1016/0304-4165(89)90087-1. PMID 2505854.
- Joziasse DH, Damen HC, de Jong-Brink M, Edzes HT, Van den Eijnden DH (August 1987). "Identification of a UDP-Gal:beta-galactoside beta 1----3-galactosyltransferase in the albumen gland of the snail Lymnaea stagnalis". FEBS Letters. 221 (1): 139–44. doi:10.1016/0014-5793(87)80368-x. PMID 3113997. S2CID 84171728.
- Weinland H (1953). "[In vitro galactogen decomposition by enzymes; studies on Helix pomatia. I. Orientation on occurrence and effect of the galactogen-splitting enzyme]". Biochemische Zeitschrift. 324 (1): 19–31. PMID 13093716.
- Myers FL, Northcote DH (1958-09-01). "A Survey of the Enzymes from the Gastro-Intestinal Tract of Helix Pomatia". Journal of Experimental Biology. 35 (3): 639–648. doi:10.1242/jeb.35.3.639. ISSN 0022-0949. Retrieved 2020-08-17.
- Dreon MS, Heras H, Pollero RJ (July 2004). "Characterization of the major egg glycolipoproteins from the perivitellin fluid of the apple snail Pomacea canaliculata". Molecular Reproduction and Development. 68 (3): 359–64. doi:10.1002/mrd.20078. PMID 15112330. S2CID 22032382.
- Heras H, Dreon MS, Ituarte S, Pollero RJ (2007-07-01). "Egg carotenoproteins in neotropical Ampullariidae (Gastropoda: Arquitaenioglossa)". Comparative Biochemistry and Physiology. Toxicology & Pharmacology. Fourth Special Issue of CBP dedicated to The Face of Latin American Comparative Biochemistry and Physiology organized by Marcelo Hermes-Lima (Brazil) and co-edited by Carlos Navas (Brazil), Rene Beleboni (Brazil), Rodrigo Stabeli (Brazil), Tania Zenteno-Savín (Mexico) and the Editors of CBP - This issue is dedicated to the memory of two exceptional men, Peter L. Lutz, one of the pioneers of comparative and integrative physiology, and Cicero Lima, journalist, science lover and Hermes-Lima's dad. 146 (1–2): 158–67. doi:10.1016/j.cbpc.2006.10.013. PMID 17320485.