Galunisertib
Galunisertib (LY2157299) is a small molecular experimental cancer drug previously in development by Eli Lilly. It is a TGF-b inhibitor.[1] Development of galunisertib by Eli Lilly was discontinued in January 2020.[2]
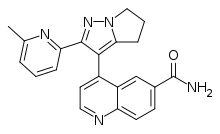 | |
| Clinical data | |
|---|---|
| Routes of administration | PO |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H19N5O |
| Molar mass | 369.428 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Galunisertib was investigated in a phase II trial for treatment of hepatocellular carcinoma.[3] Pre-clinically, combination of galunisertib with PD-L1 blockade resulted in improved tumor growth inhibition.[4]
References
- "Lilly Oncology Pipeline - Lilly USA". www.lillyoncologypipeline.com.
- "Eli Lilly cuts 3 cancer drugs amid Q4 clear-out".
- Clinical trial number NCT01246986 for "A Study of LY2157299 in Participants With Hepatocellular Carcinoma" at ClinicalTrials.gov
- Holmgaard RB, Schaer DA, Li Y, Castaneda SP, Murphy MY, Xu X, Inigo I, Dobkin J, Manro JR, Iversen PW, Surguladze D, Hall GE, Novosiadly RD, Benhadji KA, Plowman GD, Kalos M, Driscoll KE (June 2018). "Targeting the TGFβ pathway with galunisertib, a TGFβRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade". Journal for Immunotherapy of Cancer. 6 (1): 47. doi:10.1186/s40425-018-0356-4. PMC 5987416. PMID 29866156.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.