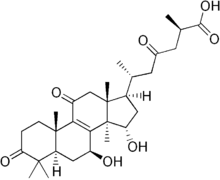
Ganoderic acid
Ganoderic acids are a class of closely related triterpenoids (derivatives from lanosterol) found in Ganoderma mushrooms. For thousands of years, the fruiting bodies of Ganoderma fungi have been used in traditional medicines in East Asia.[1][2] Consequently, there have been efforts to identify the chemical constituents that may be responsible for the putative pharmacological effects. The two most well described ganoderic acids out of the many that have been identified and characterized are ganoderic acids A and B. Some ganoderic acids have been found to possess biological activities including hepatoprotection,[3] anti-tumor effects,[4] and 5-alpha reductase inhibition.[5]
 | |
| Names | |
|---|---|
| IUPAC name
(25R)-7β,15α-Dihydroxy-3,11,23-trioxolanost-8-en-26-oic acid | |
| Systematic IUPAC name
(2R,6R)-6-[(1R,3S,3aR,4S,5aR,9aS,11aR)-3,4-Dihydroxy-3a,6,6,9a,11a-pentamethyl-7,10-dioxo-2,3,3a,4,5,5a,6,7,8,9,9a,10,11,11a-tetradecahydro-1H-cyclopenta[a]phenanthren-1-yl]-2-methyl-4-oxoheptanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C30H44O7 | |
| Molar mass | 516.67 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Nishitoba, Tsuyoshi; Goto, Sanae; Sato, Hiroji; Sakamura, Sadao (1989). "Bitter triterpenoids from the fungus Ganoderma applanatum". Phytochemistry. 28 (1): 193–7. doi:10.1016/0031-9422(89)85036-8.
- Mizuno, T.; Wang, G.; Zhang, J.; Kawagishi, H.; Nishitoba, T.; Li, J. (1995). "Reishi, Ganoderma lucidum and Ganoderma tsugae: Bioactive substances and medicinal effects". Food Reviews International. 11: 151–166. doi:10.1080/87559129509541025.

- Liu, Li-Ying; Chen, Hui; Liu, Chao; Wang, Hong-Qing; Kang, Jie; Li, Yan; Chen, Ruo-Yun (2014). "Triterpenoids of Ganoderma theaecolum and their hepatoprotective activities". Fitoterapia. 98: 254–9. doi:10.1016/j.fitote.2014.08.004. PMID 25111010.
- Wang, G.; Zhao, J.; Liu, J.; Huang, Y.; Zhong, J. J.; Tang, W. (2007). "Enhancement of IL-2 and IFN-γ expression and NK cells activity involved in the anti-tumor effect of ganoderic acid Me in vivo". International Immunopharmacology. 7 (6): 864–870. doi:10.1016/j.intimp.2007.02.006. PMID 17466920.

- Liu, J.; Kurashiki, K.; Shimizu, K.; Kondo, R. (December 2006). "Structure–activity relationship for inhibition of 5α-reductase by triterpenoids isolated from Ganoderma lucidum". Bioorganic & Medicinal Chemistry. 14 (24): 8654–8660. doi:10.1016/j.bmc.2006.08.018. PMID 16962782.

This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.