Germanate
In chemistry, germanate is a compound containing an oxyanion of germanium. In the naming of inorganic compounds it is a suffix that indicates a polyatomic anion with a central germanium atom,[1] for example potassium hexafluorogermanate, K2GeF6.[2]
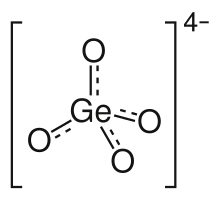
Germanate oxy compounds
Germanium is similar to silicon forming many compounds with tetrahedral {GeO4}[2] units although it can also exhibit 5[3] and 6[2] coordination. Analogues of all the major types of silicates and aluminosilicates have been prepared.[4] For example, the compounds Mg2GeO4 (olivine and spinel forms), CaGeO3(perovskite structure), Be2GeO4 (phenakite structure) show the resemblance to the silicates.[4] BaGe4O9 has a complex structure containing 4 and 6 coordinate germanium. Germanates are important for geoscience as they possess similar structures to silicates and can be used as analogues for studying the behaviour of silicate minerals found in the Earth's mantle;[5] for example, MnGeO3 has a pyroxene type structure similar to that of MgSiO3 which is a significant mineral in the mantle.[6][7][8]
Germanates in aqueous solutions
The alkali metal orthogermanates, M4GeO4, containing discrete GeO4−
4 ions, form acidic solutions containing GeO(OH)−
3, GeO
2(OH)2−
2 and [(Ge(OH)4)8(OH)3]3−.[2] Neutral solutions of germanium dioxide contain Ge(OH)4, but at high pH germanate ions such as GeO(OH)−
3, GeO
2(OH)2−
2 are present.[9]
Germanate zeolites
Microporous germanate zeolites were first prepared in the 1990s.[10][11] A common method of preparation is hydrothermal synthesis using an organic amine as a template (structure determining agent).[12] The frameworks are negatively charged due to extra oxide ions which leads to higher coordination numbers for germanium of 5 and 6. The negative charge is balanced by the positively charged amine molecules.
In addition to the ability of germanium to exhibit 4, 5 or 6 coordination, the greater length of the Ge–O bond in the {GeO4} tetrahedral unit compared to Si–O in {SiO4} and the narrower Ge–O–Ge angle (130°–140°) between corner shared tetrahedra allow for unusual framework structures.[13] A zeolite reported in 2005[14] has large pores – 18.6 × 26.2 Å interconnected by channels defined by 30-membered rings (the naturally occurring zeolite faujasite with channels defined by 12-membered rings[15]). Zeolites with frameworks containing silicon and germanium (silicogermanates), aluminium and germanium (aluminogermanates) and zirconium and germanium (zirconogermanates) are all known.[12][16]
See also
- Bismuth germanate (bismuth germanium oxide, BGO)
- Sodium germanate
References
- Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005 – Full text (PDF)
- Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0123526515
- Nguyen, Quang Bac; Lii, Kwang-Hwa (2011). "Cs4UGe8O20: A Tetravalent Uranium Germanate Containing Four- and Five-Coordinate Germanium". Inorganic Chemistry. 50 (20): 9936–9938. doi:10.1021/ic201789f. ISSN 0020-1669. PMID 21939186.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Ringwood, A.E. (1970). "Phase transformations and the constitution of the mantle". Physics of the Earth and Planetary Interiors. 3: 109–155. Bibcode:1970PEPI....3..109R. doi:10.1016/0031-9201(70)90047-6. ISSN 0031-9201.
- Ringwood, A. E.; Seabrook, Merren (1962). "Some High-pressure Transformations in Pyroxenes". Nature. 196 (4857): 883–884. Bibcode:1962Natur.196..883R. doi:10.1038/196883a0. ISSN 0028-0836. S2CID 4183493.
- Hirose, Kei; Nagaya, Yukio; Merkel, Sébastien; Ohishi, Yasuo (2010). "Deformation of MnGeO3 post-perovskite at lower mantle pressure and temperature". Geophysical Research Letters. 37 (20). Bibcode:2010GeoRL..3720302H. doi:10.1029/2010GL044977. ISSN 0094-8276.
- Matsumura, Hisashi; Mamiya, Mikito; Takei, Humihiko (2000). "Growth of pyroxene-type MnGeO3 and (Mn,Mg)GeO3 crystals by the floating-zone method". Journal of Crystal Growth. 210 (4): 783–787. Bibcode:2000JCrGr.210..783M. doi:10.1016/S0022-0248(99)00850-7. ISSN 0022-0248.
- "Germanium: Inorganic Chemistry" F Glockling Encyclopedia of Inorganic Chemistry Editor R Bruce King (1994) John Wiley and Sons ISBN 0-471-93620-0
- Cheng, Jun; Xu, Ruren; Yang, Guangdi (1991). "Synthesis, structure and characterization of a novel germanium dioxide with occluded tetramethylammonium hydroxide". Journal of the Chemical Society, Dalton Transactions (6): 1537. doi:10.1039/dt9910001537. ISSN 0300-9246.
- Li, Hailian; Yaghi, O. M. (1998). "Transformation of Germanium Dioxide to Microporous Germanate 4-Connected Nets". Journal of the American Chemical Society. 120 (40): 10569–10570. doi:10.1021/ja982384n. ISSN 0002-7863.
- Zeolites and Related Materials: Trends Targets and Challenges(SET), 1st Edition, 4th International FEZA Conference, 2008, Paris, France; Eds. Gedeon, Massiani, Babonneau; Elsevier Science; ISBN 9780444532961
- Introduction to Zeolite Molecular Sieves, Jiri Cejka, Herman van Bekkum, A. Corma, F. Schueth, Elsevier, 2007
- Zou, Xiaodong; Conradsson, Tony; Klingstedt, Miia; Dadachov, Mike S.; O'Keeffe, Michael (2005). "A mesoporous germanium oxide with crystalline pore walls and its chiral derivative". Nature. 437 (7059): 716–719. Bibcode:2005Natur.437..716Z. doi:10.1038/nature04097. ISSN 0028-0836. PMID 16193048. S2CID 4411828.
- Handbook Of Molecular Sieves: Structures, Rosemarie Szostak, 1992, Van Nostrand Reinhold, ISBN 0442318995, ISBN 978-0442318994
- Plévert, Jacques; Sanchez-Smith, Rebeca; Gentz, Travis M.; Li, Hailian; Groy, Thomas L.; Yaghi, Omar M.; O'Keeffe, Michael (2003). "Synthesis and Characterization of Zirconogermanates". Inorganic Chemistry. 42 (19): 5954–5959. doi:10.1021/ic034298g. ISSN 0020-1669. PMID 12971765.