Glutathione-ascorbate cycle
The ascorbate-glutathione cycle, sometimes Foyer-Halliwell-Asada pathway, is a metabolic pathway that detoxifies hydrogen peroxide (H2O2), a reactive oxygen species that is produced as a waste product in metabolism. The cycle involves the antioxidant metabolites: ascorbate, glutathione and NADPH and the enzymes linking these metabolites.[1]
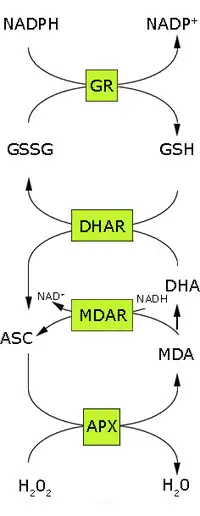
In the first step of this pathway, H2O2 is reduced to water by ascorbate peroxidase (APX) using ascorbate (ASC) as the electron donor. The oxidized ascorbate (monodehydroascorbate, MDA) is regenerated by monodehydroascorbate reductase (MDAR).[2] However, monodehydroascorbate is a radical and if not rapidly reduced it disproportionates into ascorbate and dehydroascorbate (DHA). Dehydroascorbate is reduced to ascorbate by dehydroascorbate reductase (DHAR) at the expense of GSH, yielding oxidized glutathione (GSSG). Finally GSSG is reduced by glutathione reductase (GR) using NADPH as the electron donor. Thus ascorbate and glutathione are not consumed; the net electron flow is from NADPH to H2O2. The reduction of dehydroascorbate may be non-enzymatic or catalysed by proteins with dehydroascorbate reductase activity, such as glutathione S-transferase omega 1 or glutaredoxins.[3][4]
In plants, the glutathione-ascorbate cycle operates in the cytosol, mitochondria, plastids and peroxisomes.[5][6] Since glutathione, ascorbate and NADPH are present in high concentrations in plant cells it is assumed that the glutathione-ascorbate cycle plays a key role for H2O2 detoxification. Nevertheless, other enzymes (peroxidases) including peroxiredoxins and glutathione peroxidases, which use thioredoxins or glutaredoxins as reducing substrates, also contribute to H2O2 removal in plants.[7]
See also
References
- Noctor G, Foyer CH (Jun 1998). "ASCORBATE AND GLUTATHIONE: Keeping Active Oxygen Under Control". Annu Rev Plant Physiol Plant Mol Biol. 49: 249–279. doi:10.1146/annurev.arplant.49.1.249. PMID 15012235.
- Wells WW, Xu DP (August 1994). "Dehydroascorbate reduction". J. Bioenerg. Biomembr. 26 (4): 369–77. doi:10.1007/BF00762777. PMID 7844111. S2CID 24723138.
- Whitbread AK, Masoumi A, Tetlow N, Schmuck E, Coggan M, Board PG (2005). "Characterization of the omega class of glutathione transferases". Meth. Enzymol. Methods in Enzymology. 401: 78–99. doi:10.1016/S0076-6879(05)01005-0. ISBN 9780121828066. PMID 16399380.
- Rouhier N, Gelhaye E, Jacquot JP (2002). "Exploring the active site of plant glutaredoxin by site-directed mutagenesis". FEBS Lett. 511 (1–3): 145–9. doi:10.1016/S0014-5793(01)03302-6. PMID 11821065. S2CID 29816004.
- Meyer A (Sep 2009). "The integration of glutathione homeostasis and redox signaling". J Plant Physiol. 165 (13): 1390–403. doi:10.1016/j.jplph.2007.10.015. PMID 18171593.
- Jimenez A, Hernandez JA, Pastori G, del Rio LA, Sevilla F (Dec 1998). "Role of the Ascorbate-Glutathione Cycle of Mitochondria and Peroxisomes in the Senescence of Pea Leaves". Plant Physiol. 118 (4): 1327–35. doi:10.1104/pp.118.4.1327. PMC 34748. PMID 9847106.
- Rouhier N, Lemaire SD, Jacquot JP (2008). "The role of glutathione in photosynthetic organisms: emerging functions for glutaredoxins and glutathionylation" (PDF). Annu Rev Plant Biol. 59: 143–66. doi:10.1146/annurev.arplant.59.032607.092811. PMID 18444899.