Glutaurine
Glutaurine is an endogenous dipeptide which is an amide formed from glutamic acid and taurine.
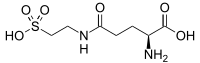 | |
| Names | |
|---|---|
| IUPAC name
N5-(2-Sulfoethyl)-L-glutamine | |
| Systematic IUPAC name
(2S)-2-Amino-5-oxo-5-[(2-sulfoethyl)amino]pentanoic acid | |
| Other names
γ-Glutamyltaurine; γ-GT; γ-L-Glutamyltaurine[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H14N2O6S | |
| Molar mass | 254.26 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Biological role
Glutaurine is an antiepileptic with antiamnesia properties. Glutaurine was discovered in the parathyroid in 1980, and later in the mammalian brain. This led to studies on intrinsic and synthetic taurine peptides, and the suggestion that γ-glutamyltransferase (GGT; γ-glutamyl-transpeptidase) in the brain is responsible for its in vivo formation.[2]
The versatile molecule mimics the anxiolytic drug diazepam, and is implicated in phenomena from feline aggression to amphibian metamorphosis, radiation protection, and the glutamatergic system in schizophrenic disorders.[2]
References
- "56488-60-9 CAS Manufactory". Chemicalbook.com. Retrieved 2012-04-21.
- Bittner, S.; Win, T.; Gupta, R. (2005). "γ-L-glutamyltaurine". Amino Acids. 28 (4): 343–356. doi:10.1007/s00726-005-0196-7. PMID 15838590. S2CID 209532290.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.